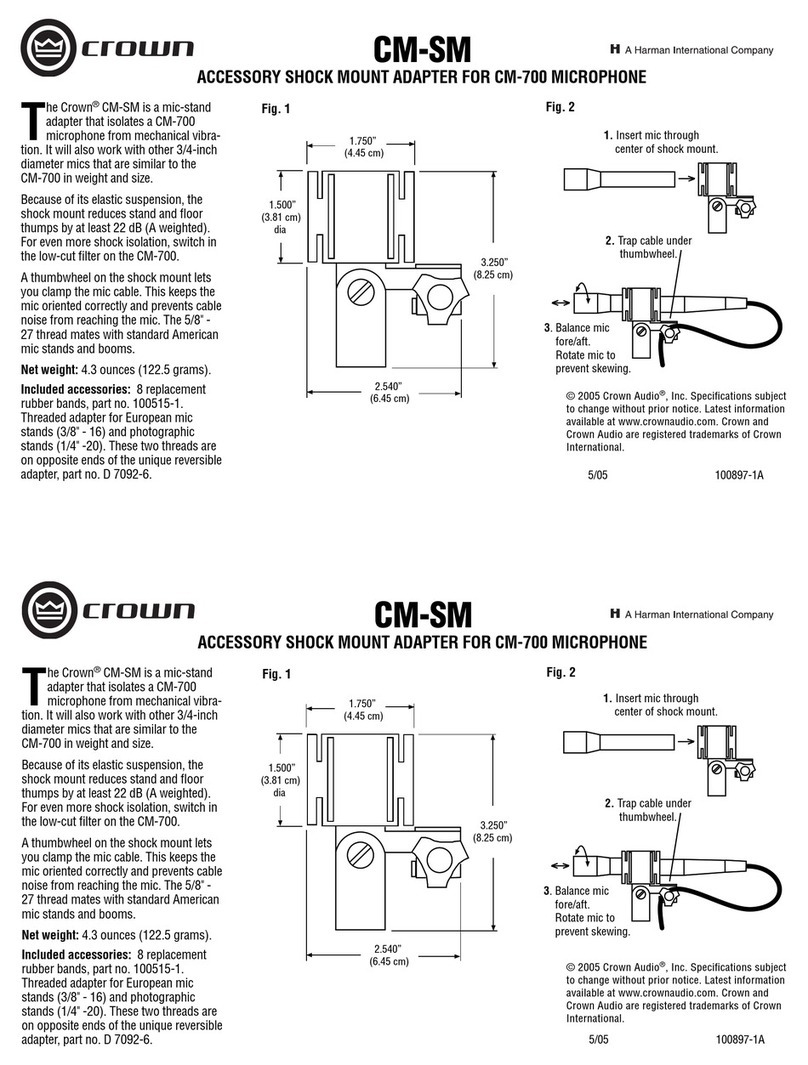
Instrumentation Industries RTC 15-D Setup guide

Product Specifications
Sterilization / Disinfection
Always carefully examine the physical integrity of this product after processing. Do not use if cracking or crazing is seen,
or if parts fit together improperly after processing.
RTC 22-A — RTC 23-A
These products are designed to be Reusable. (May be processed up to 30 times at the parameters provided below.)
They are packaged as non-sterile; Instrumentation Industries, Inc. recommends processing before initial use.
Reprocess when replacing the circuit at the parameters provided below.
Recommendations for RTC 22-A and RTC 23-A:
Carefully follow facility guidelines for preparing products and solutions for the sterilizing and disinfecting methods and
equipment being used. Sterilization and High Level Disinfection Procedures have been validated for the following equipment
and methods:
Sterilization
Steam Autoclave
Do not exceed 135°C/275°F.
High Level Disinfection
Pasteurization
Pasteurize at 70°C +/- 3°C (153° - 163°F) for a minimum of 30 minutes.
Cold Chemicals
Recommended Chemical: 2-4% Activated Glutaraldehyde
Disinfect according to validated parameters. Follow the chemical manufacturer’s recommendation for temperature and soak
time. Chemical disinfection should be followed by sterile water rinse. Exposure time should be based on the manufacturer’s
indication for use as a high-level disinfectant or sterilant.
Do not use alcohol or chemicals containing dimethyl ammonium chloride.
Use: Reusable
Inlet: 22mm I.D.
Outlet: 15mm I.D./22mm O.D.
Length: 2”
Materials
Body: Polyetherimide
Cap: Silicone Rubber
Color
Body: Amber
Cap: Clear
Use: Single Patient Reuse
(see Panel A for info)
Inlet: 22mm I.D.
Outlet: 15mm I.D./22mm O.D.
Length: 2”
Materials
Body: Polystyrene Butadiene
Cap: Thermoplastic Rubber
Color
Body: Clear
Cap: Clear
Use: Reusable
Inlet: 15mm I.D./22mm O.D.
Outlet: 15mm I.D./22mm O.D.
Length: 2”
Materials
Body: Polyetherimide
Cap: Silicone Rubber
Color
Body: Amber
Cap: Clear
Use: Single Patient Reuse
(see Panel A for info)
Inlet: 15mm I.D./22mm O.D.
Outlet: 15mm I.D./22mm O.D.
Length: 2”
Materials
Body: Polystyrene Butadiene
Cap: Thermoplastic Rubber
Color
Body: Clear
Cap: Clear
RTC 15-D
Metered
Dose Inhaler
(MDI) Adapter
RTC 15-P
Metered
Dose Inhaler
(MDI) Adapter
Use: Single Patient Reuse
(see Panel A for info)
Inlet: 15mm I.D.
Outlet:15mm I.D.
Length: 2”
Materials
Body: Polystyrene Butadiene
Cap: Thermoplastic Rubber
Color
Body: Clear
Cap: Clear
RTC 22-A
Metered
Dose Inhaler
(MDI) Adapter
RTC 22-D
Metered
Dose Inhaler
(MDI) Adapter
RTC 23-A
Metered
Dose Inhaler
(MDI) Adapter
RTC 23-D
Metered
Dose Inhaler
(MDI) Adapter
MDI
Adapter
Installation &
Usage
Directions
RTC 15-D
RTC 15-P
RTC 22-A
RTC 22-D
RTC 23-A
RTC 23-D
For use with metered dose inhaler
canisters, with standard size metal
(.109 inch diameter) or plastic
(.124 inch diameter) stems.
Instrumentation
Industries, Inc.
Instrumentation
Industries, Inc.
2990 Industrial Blvd.
Bethel Park, PA 15102
US Toll Free: 1-800-633-8577
Business: 1-412-854-1133
US Toll Free Fax: 1-877-633-8661
Fax: 1-412-854-5668
E-mail: [email protected]
www.iiimedical.com ECO 2526 Rev. J2-18
— Not made with Natural Rubber Latex
— Not made with Di(2-ethylhexyl) phthalate (DEHP)
Use: Single Patient Reuse
(see Panel A for info)
Inlet: 15mm O.D.
Outlet: 15mm I.D.
Length: 2"
Materials
Body: Polystyrene Butadiene
Cap: Thermoplastic Rubber
Color
Body: Clear
Cap: Clear
G H
RTC 15-D — RTC 15-P — RTC 22-D — RTC 23-D
These products are designed for Single Patient Reuse.(Can be used to repeatedly administer medications to one single patient
while installed in a breathing circuit.)
They are packaged as non-sterile; Instrumentation Industries, Inc. recommends processing before initial use.
Discard when replacing the circuit according to facility guidelines.
Recommendations for RTC 15-D, RTC 15-P, RTC 22-D, and RTC 23-D:
Carefully follow facility guidelines for preparing products and solutions for the sterilizing and disinfecting methods and
equipment being used. Sterilization and High Level Disinfection Procedures have been validated for the following equipment
and methods:
Sterilization
STERRAD 100S
Full short sterilization cycle (Hydrogen peroxide gas plasma, 55 minutes, 45°C - 55°C (113°F - 131°F)).
High Level Disinfection
Pasteurization
Pasteurize at 70°C +/- 3°C (153° - 163°F) for a minimum of 30 minutes.
CIDEX®OPA
Prepare CIDEX®OPA solution. Totally submerge adapter in the disinfectant. Immerse device completely, eliminating air
pockets, in CIDEX®OPA solution for a minimum of 12 minutes at 20°C (68°F) to destroy all pathogenic microorganisms.
Remove device from the solution and rinse thoroughly as per these rinsing instructions:
• Following removal from CIDEX®OPA solution, thoroughly rinse the adapter by immersing it completely in a large volume
(e.g. 2 gallons) of water.
Sterile water rinse is recommended unless potable water is acceptable.
• Keep the device totally immersed for a minimum of 1minute in duration.
• Manually flush all devices with large volumes (not less than 100 mL) of rinse water.
• Remove the device and discard the rinse water. Always use fresh volumes of water for each rinse.
Do not reuse the water for rinsing or any other purpose.
• Repeat the procedure two (2) additional times, for a total of three (3) rinses, with large volumes of fresh water to remove
CIDEX®OPA solution residues. Residues may cause serious side effects.
• Dry using sterile, lint-free cloths.
DO NOT STEAM AUTOCLAVE RTC 15-D — RTC 15-P — RTC 22-D — RTC 23-D!
Visit iiimedical.com/symbols.pdf
for the Glossary of Symbols used in
Instrumentation Industries, Inc. labeling. Made in USA
These user instructions are available in Canadian French
at iiimedical.com
Ces instructions d’utilisation sont disponibles en français
canadien à iiimedical.com

Inspiratory Limb
RTC 15-P
MDI Adapter
Outlet
Endotracheal
Tube
15mm
Patient Wye
*Note:
Inspiratory Limb
tubing must have a
15mm O.D. x 15mm O.D.
adapter (not included)
to attach to RTC 15-P.
Inlet
Expiratory Limb
Rubber Cap
3
1
2
RTC 15-D/P —RTC 22-A/D — RTC 23-A/D Installation Instructions
BBBBB
A
Inspiratory Limb
Expiratory Limb
RTC 15-D
MDI Adapter
Outlet Endotracheal
Tube
15mm
Patient Wye
Inlet
Rubber Cap
3
12
Installation Instructions
Canister Insertion
Medication
Direction
Arrow Rubber
Cap
Removed
Pressurized
Medicine
Canister
Canister
Activator
Canister
Activator
Port
Indications for Use
Notes
Contraindications
A
B
D
E
F
C
The Instrumentation Industries, Inc. RTC 15-D, RTC 15-P, RTC 22-A, RTC 22-D, RTC 23-A, and RTC 23-D Adapters are actuators for intermittent delivery of prescribed aerosol medication dispensed
in metered dose inhalers. RTC 15-D, RTC 15-P, RTC 22-A, RTC 22-D, RTC 23-A, and RTC 23-D Adapters are intended for use only when connected to ventilator tubing or tracheal tubes.
The RTC 15-D, RTC 15-P, RTC 22-D, and RTC 23-D Adapters are intended for Single Patient Reuse. (Can be used to repeatedly administer medications to one single patient while installed in a breathing
circuit.) RTC 22-A and RTC 23-A adapters are intended to be Reusable. These devices are intended for sale by or on the order of a physician.
— All illustrations are examples.
— Consult facility-validated procedures for desired placement.
None known. — Additional adapters or connectors may be needed in certain placements.
— In all set-ups, direction arrow must be pointing in the direction of airflow.
Canister Activation
RTC 23-A/D
MDI Adapter
Inspiratory Limb
Expiratory Limb
Inlet Outlet
Rubber Cap Endotracheal
Tube
Patient
Wye
**Note: To attach RTC 23-A/D to
patient wye, a 22mm I.D. x 22mm I.D.
Adapter (not included) is needed.
3
1
2
1. Connect RTC 23-A/D Inlet
to Inspiratory Limb of
Patient Circuit, as shown.
2. Connect RTC 23-A/D Outlet to
Patient Wye.**
3. Ensure that Rubber Cap is placed on
MDI Activator Port until MDI canister
is inserted.
1. Connect RTC 22-A/D Inlet to Patient Wye, as shown.
2. Connect RTC 22-A/D Outlet to Endotracheal Tube.
3. Ensure that Rubber Cap is placed on MDI Activator Port until MDI canister is inserted.
1. Connect RTC 15-P Inlet
to Inspiratory Limb of
Patient Circuit, as shown.*
2. Connect RTC 15-P Outlet
to Patient Wye.
3. Ensure that Rubber Cap is placed
on MDI Activator Port until MDI
canister is inserted.
1. Connect RTC 15-D Inlet to Patient Wye, as shown.
2. Connect RTC 15-D Outlet to Endotracheal Tube.
3. Ensure that Rubber Cap is placed on MDI Activator Port until MDI canister is inserted.
RTC 15-D — Placed at Endotracheal Tube
1
1
1
1
RTC 15-P — Placed in Inspiratory Limb
2
2
2
2
3
3
3
3
RTC 22-A/D — Placed at Endotracheal Tube RTC 23-A/D — Placed in Inspiratory Limb
Inspiratory Limb
Expiratory Limb
Endotracheal
Tube
Patient
Wye RTC 22-A/D
MDI Adapter
Inlet Outlet
Rubber Cap
3
12
1. Install MDI
Adapter in
Patient Circuit.
(Panel D)
2. Remove Rubber
Cap from
MDI Adapter
Canister
Activator Port.
3. Insert
Pressurized
Medicine
Canister
into
Canister
Activator
Port.
Medication
Direction
Arrow
Rubber
Cap
Removed
Medication
directed
mainstream
• Prior to activation, make sure that the Pressurized
Medicine Canister is fully seated in MDI Adapter.
• While activating the Pressurized Medicine Canister,
press the canister straight down without tilting.
• Two hands may be necessary to keep canister
aligned properly.
1. At the beginning of
the inspiratory cycle,
depress the
Pressurized
Medicine Canister
by placing fingers
underneath MDI
Adapter and pressing
top of canister
with thumb.
2. Remove
Canister from
MDI Adapter
Canister
Activator Port.
3. Replace Rubber Cap.
This manual suits for next models
5