1 User instructions ........................................................................................................................................ 2
2 Safety ........................................................................................................................................................ 3
2.1 Description of safety instructions ..................................................................................................... 3
2.1.1 Warning symbol .................................................................................................................. 3
2.1.2 Structure ............................................................................................................................. 3
2.1.3 Description of danger levels ............................................................................................... 3
2.2 Purpose – Proper use ...................................................................................................................... 3
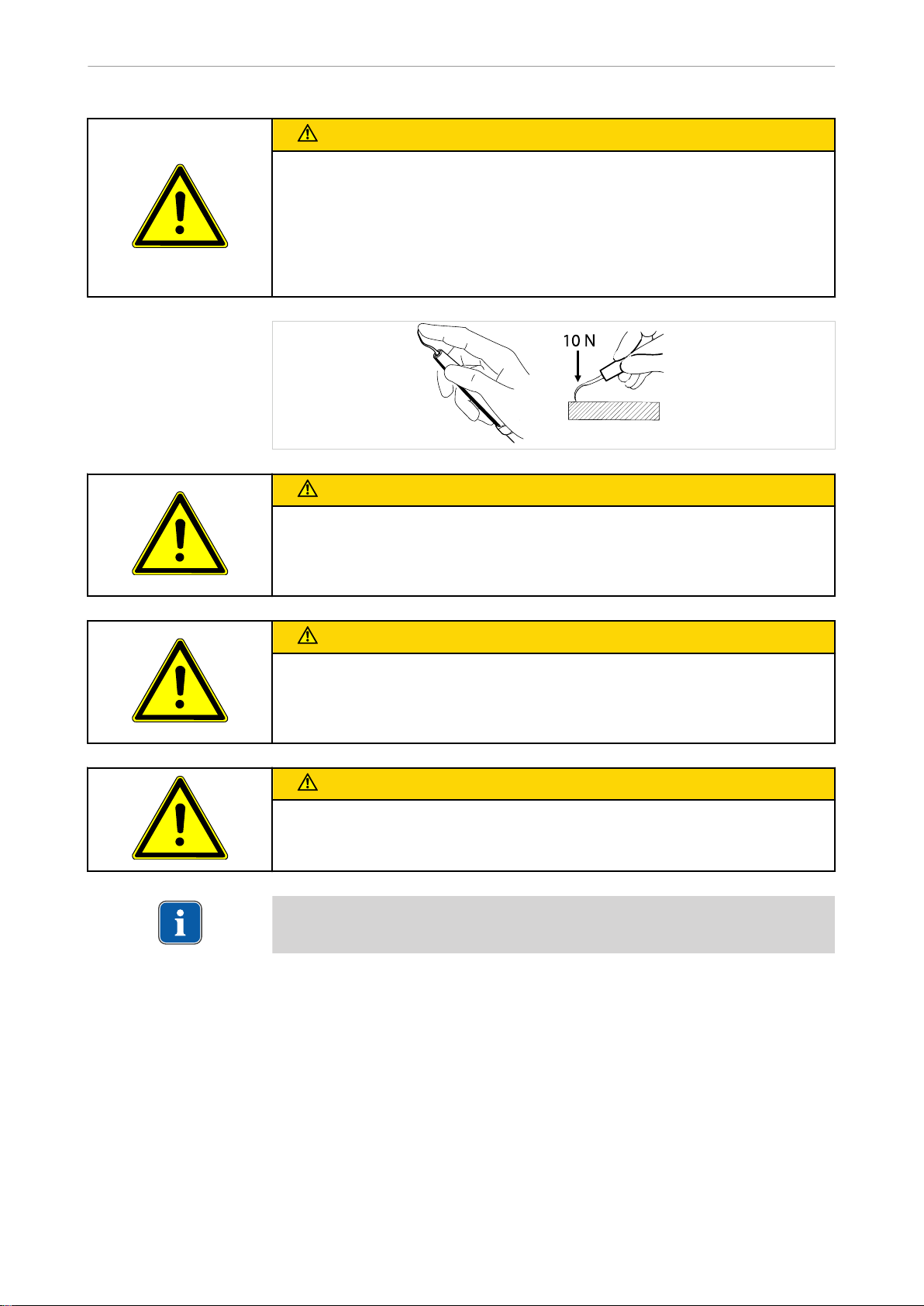
2.3 Safety instructions ........................................................................................................................... 4
3 Product description .................................................................................................................................... 6
3.1 Technical data ................................................................................................................................. 6
3.1.1 Identification tip type ........................................................................................................... 6
3.2 Transportation and storage conditions ............................................................................................ 6
4 First use ..................................................................................................................................................... 8
4.1 Inserting the SONICflex tips ............................................................................................................ 8
4.2 Removing the SONICflex tip ........................................................................................................... 8
5 Operation ................................................................................................................................................... 9
5.1 Power settings for the SONICflex ................................................................................................... 9
5.2 Instructions for use .......................................................................................................................... 9
6 Preparation methods according to ISO 17664 ........................................................................................ 12
6.1 Preparation at the site of use ........................................................................................................ 12
6.2 Preparation before cleaning .......................................................................................................... 12
6.3 Cleaning ........................................................................................................................................ 12
6.3.1 Manual cleaning of the exterior ....................................................................................... 13
6.3.2 Manual cleaning of the interior ......................................................................................... 13
6.3.3 Mechanical cleaning of the exterior and interior .............................................................. 13
6.4 Disinfection .................................................................................................................................... 13
6.4.1 Manual disinfection of the the exterior ............................................................................. 14
6.4.2 Manual disinfection of the interior ..................................................................................... 14
6.4.3 Mechanical disinfection of the exterior and interior .......................................................... 14
6.5 Drying ........................................................................................................................................... 15
6.6 Packaging ...................................................................................................................................... 15
6.7 Sterilisation .................................................................................................................................... 15
6.8 Storage .......................................................................................................................................... 16
7 Tools ........................................................................................................................................................ 17
Instructions for use For SONICflex tips paro - REF 0.571.0371, paro A - REF 1.006.2020
1/17