8
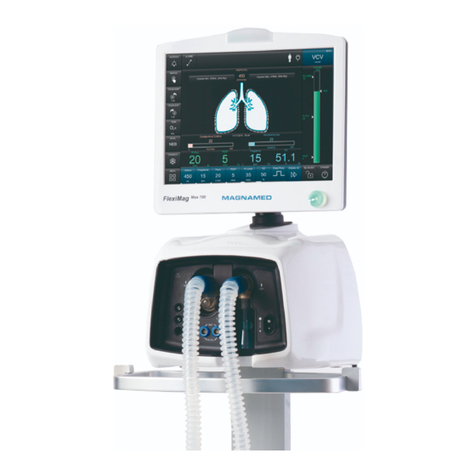
MAGNAMED TECNOLOGIA MÉDICA S/A
operation may be compromised.
•During the equipment’s prolonged use in patients with excess secretion or in breathing
system using a heated humidifier, the flow sensor’s condition must be frequently verified.
•The equipment has an independent power supply and its own battery backup system.
•Connect an AC power cord to a three pin socket NBR 14136:2002 (2P+T);
•Maintain the equipment connected to a power source even when it is turned off in order to
maintain the internal batteries permanently charged;
•Completely recharge the batteries after use or after a long stocking period;
•The alarm battery recharge must be promptly attended to. Perform your recharge before
the next use of the equipment, because any power outage can stop the operation.
•If after a long period of time using battery, occurs a LOW BATTERY alarm, provide an
IMMEDIATE connection of the power cord to a power supply, I it is not possible, provide
adequate ventilator support means and DISCONNECT the patient from ventilator.
•The absence of obstruction is extremely important for the correct operation of ventilation
monitoring. Therefore, it must be frequently verified during the patient’s ventilation
realization.
•After usage, the ventilator breathing system components MUST be disinfected before their
next used, whenever the same are reusable.
•All of the equipment’s parts that came into contact with fluids from the patients must
undergo a high-level disinfection process or sterilization when discarded or be discarded
as potentially infected medical waste.
•All parts applied of FlexiMag ventilators are made of nontoxic material, they are exempted
of latex and do not cause irritation or allergy to the patient (biocompatibility).
•The common use accessories, which are not exclusive to FlexiMag, such as: masks,
respiratory circuits, nebulizers, heated humidifiers, HME filters, among others, must
comply with local legal government requirements.
•Do not use the equipment if the problem cannot be solved.
•Have a ventilation manual powered available, to use in the cases of: the battery to be
completely depleted or, there is a lack of gas for the ventilator operation; or general failure
of the ICU ventilator.
•Always use officially approved oxygen cylinders and pressure redactor valves that attend
to local legal government requirements.
•In order for appropriate ventilation, take in account the ventilator breathing system’s dead