3 Manual Oxymag_Rev22
Analytical Index
A.DEFINITIONS ..................................................... 5
B.WARNING ........................................................ 5
C.CAUTION.......................................................... 7
D.NOTES ............................................................. 7
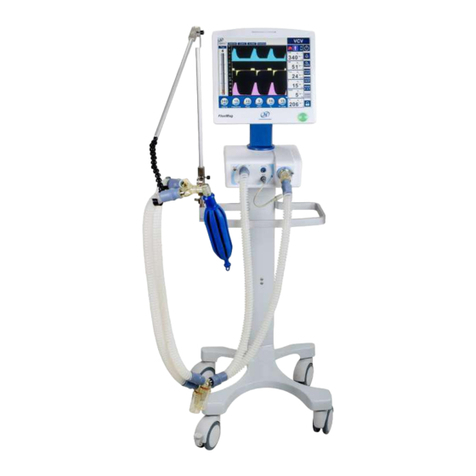
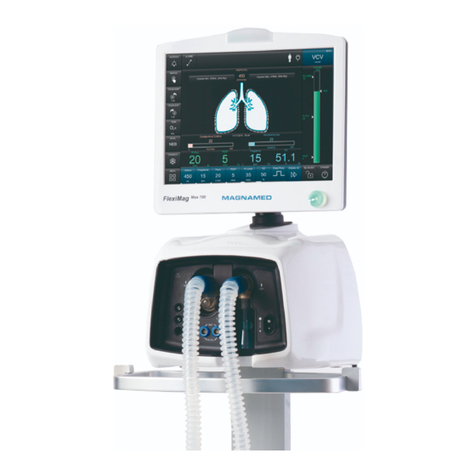
1. DESCRIPTION .................................................. 8
1.1 INTENDED USE .................................................. 8
1.2 OPTIONAL ITEMS COMPATIBLE WITH THE PRODUCTS.. 9
2. UNPACKING THE PRODUCT ........................... 10
2.1 INITIAL CHECKS ............................................... 10
2.2 PARTS AND ACCESSORIES................................. 11
2.3 OPTIONAL ACCESSORIES THAT CAN BE PURCHASED
FOR OXYMAG.................................................................... 12
2.4 IDENTIFICATION OF COMPONENTS....................... 15
COMPONENTS OF TRANSPORT VENTILATOR....... 15
3. DESCRIPTION OF THE DISPLAY...................... 18
3.1 MODES .......................................................... 18
3.2 ALARMS,MONITOR AND STATUS ......................... 18
3.3MONITOR,MENUS AND CHARTS.......................... 18
3.4 SETTING THE VENTILATION PARAMETERS .............. 18
4. PREPARATION FOR USE ................................ 19
4.1 ASSEMBLING OXYMAG –TRANSPORT VENTILATION 19
4.2 NONINVASIVE VENTILATION MASK ........................ 21
4.3 POWER CONNECTION ....................................... 22
4.4 MOUNTING THE VERTICAL SUPPORT..................... 22
5. CHECKS BEFORE USE ................................... 24
5.1 INITIAL PROCEDURES ........................................ 24
5.2 VENTILATOR SETTINGS ..................................... 25
NORMAL STARTUP SEQUENCE ....................... 27
TEST SEQUENCE ......................................... 30
FAILURE DIAGNOSIS..................................... 31
6. CAPNOGRAPHY SENSOR (ETCO2).................. 32
6.1 INSTRUCTIONS FOR USE.................................... 32
6.2 ASSEMBLING THE SENSOR................................. 32
6.3 POSITIONING THE SENSOR................................. 35
6.4 PROCEDURE TO RESET THE SENSOR................... 35
6.5 INFORMATION REGARDING LED .......................... 36
6.6 PREVENTIVE MAINTENANCE OF ETCO2SENSOR .... 36
6.7 TECHNICAL SPECIFICATIONS OF THE CAPNOGRAPHY37
7. OXIMETER (MASIMO) ..................................... 41
7.1 OPERATION PRINCIPLE ..................................... 41
7.2 SENSOR ASSEMBLY ........................................... 44
8. DESCRIPTION OF MODES............................... 45
8.1 VCV –VOLUME CONTROLLED VENTILATION ..........45
8.2 PCV –PRESSURE CONTROLLED VENTILATION.........47
8.3 PLV –LIMITED PRESSURE VENTILATION.................49
8.4 V-SIMV –SYNCHRONIZED INTERMITTENT
MANDATORY VENTILATION –VOLUME CONTROLLED CYCLE .......51
8.5 P-SIMV –SYNCHRONIZED INTERMITTENT
MANDATORY VENTILATION –PRESSURE CONTROLLED CYCLE......53
8.6 CPAP/PSV –CONTINUOUS PRESSURE VENTILATION
WITH PRESSURE SUPPORT ....................................................55
8.7 DUALPAP –BI-LEVEL CONTINUOUS POSITIVE AIRWAY
PRESSURE VENTILATION ......................................................57
8.8 APRV –AIRWAY PRESSURE RELEASE VENTILATION
(MODE OBTAINED WITH INVERTED RATIO IN DUALPAP)............59
9. ALARMS AVAILABLE ......................................61
9.1 DESCRIPTION OF ALARM CONTROL .......................61
9.2 SETTING ALARMS..............................................67
9.3 MANUAL VENTILATION OF THE PATIENT .................68
9.4 ALARM TEST ....................................................68
HIGH PRESSURE ALARM:................................68
AC INPUT FAIL ALARM:...................................68
FIO2 ALARM:...............................................68
10. CLEANING AND STERILIZATION ......................70
10.1 EQUIPMENT CLEANING...................................70
EXTERNAL VENTILATOR SURFACES...................70
RESPIRATORY CIRCUIT,PROXIMAL FLOW SENSOR
AND EXHALATION VALVE.......................................................70
10.1.2.1 WASH....................................................70
10.1.2.2 RINSE ....................................................70
10.1.2.3 DRYING..................................................71
10.2 DISINFECTION ..............................................71
EXTERNAL PARTS.........................................71
RESPIRATORY CIRCUIT,EXHALATION VALVE,
PROXIMAL FLOW SENSOR AND SILICONE LINE............................71
10.3 STERILIZATION .............................................71
10.4 PROCESSING METHODS .................................72
11. PREVENTIVE MAINTENANCE..........................73
11.1 INDICATION OF THE NEED FOR PERIODIC
MAINTENANCE 73
11.2 DAILY CHECKS AND/OR PRIOR TO USE...............73
11.3 INTERNAL LITHIUM BATTERY ...........................73
11.4 INTERNAL SENSOR OF O2 CONCENTRATION ......74
11.5 REPLACING THE AMBIENT AIR FILTER ...............75
11.6 FORWARDING THE PRODUCT TO REPAIR SERVICE
76
12. DISPOSAL ......................................................77
13. TURNING OFF THE EQUIPMENT ......................78