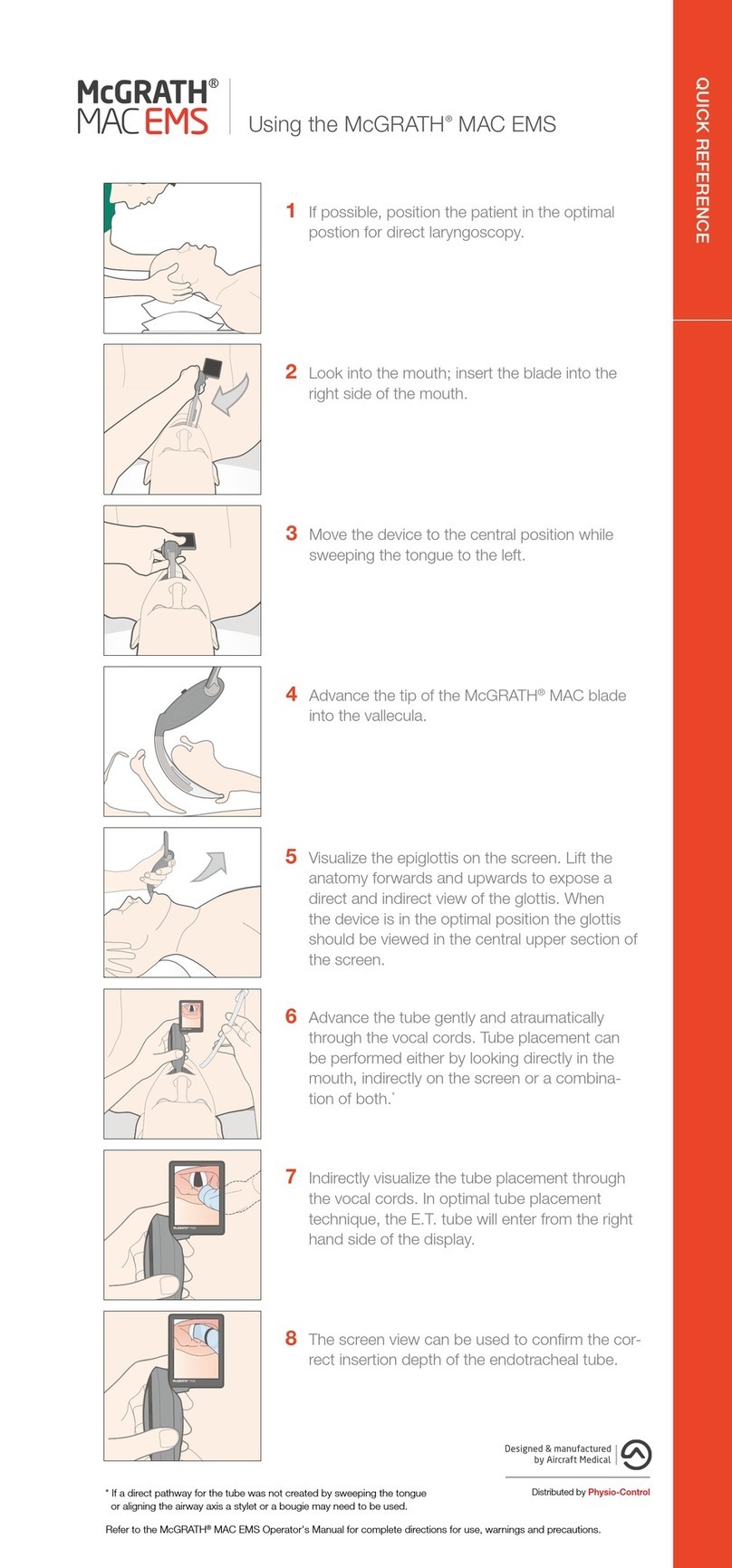
Doc no: PT00098114 Revision A Aircraft Medical Ltd © 2019 www.medtronic.com/mac-first
Manufacturer’s Declaration
The McGRATH® MAC Video Laryngoscope (the device) has been tested to product specific standard IEC 60601-1-2:2014
[Medical electrical equipment - Part 1-2: General requirements for basic safety and essential performance - Collateral Standard:
Electromagnetic disturbances - Requirements and tests].
In addition, specific requirements of standard IEC 60601-2-18:2009 [Medical electrical equipment - Part 2-18: Particular
requirements for the basic safety and essential performance of endoscopic equipment] have been applied that relate to
IEC 60601-1-2:2014.
The following tables contain the corresponding manufacturer’s declaration for the electromagnetic emissions and
electromagnetic immunity of the device.
Table 1 - Electromagnetic Emissions
The device is intended for use in the electromagnetic environment specified below. The operator of the device should
assure that it is used in such an environment.
Emissions Test Compliance Level Notes
Conducted RF emissions CISPR 11:2009
+A1:2010
Not Applicable Test not applicable as the device is
battery powered
Radiated RF emissions CISPR 11:2009
+A1:2010
Group 1 Class B Compliant
Harmonic emissions IEC 61000-3-2:2005
+A1:2008+A2:2009
Not Applicable Test not applicable as the device is
battery powered
Voltage fluctuations/flicker
IEC 61000-3-3:2008
Not Applicable Test not applicable as the device is
battery powered
Table 2 - Electromagnetic Immunity
The device is intended for use in the electromagnetic environment specified below. The operator of the device should
assure that it is used in such an environment.
Immunity test Compliance level Notes
Electrostatic Discharge (ESD)
IEC 61000-4-2:2008
±2 kV, ±4 kV, ±6 kV and ±8 kV contact
±2 kV, ±4 kV, ±8 kV and ±15 kV air
Compliant
Radiated RF Immunity
IEC 61000-4-3:2006 +A1:2007+A2:2010
10 V/m Modulation of 80% AM @
1 kHz. Frequency 80 MHz - 2.7 GHz
Compliant
Electrical Fast Transient Bursts
IEC 61000-4-4:2012
Not Applicable Test not applicable as the device is
battery powered
Surge Immunity
IEC 61000-4-5:2014
Not Applicable Test not applicable as the device is
battery powered
Conducted RF Immunity
IEC 61000-4-6:2013
Not Applicable Test not applicable as the device is
battery powered
Magnetic Immunity
IEC 61000-4-8:2009
30 A/m Compliant
Voltage Dips
IEC 61000-4-11:2004
Not Applicable Test not applicable as the device is
battery powered
1