MEDlight OCTAderm Parts list manual

OCTAderm
USER MANUAL &TECHNICAL DESCRIPTION

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 2 of 44
Table of Contents
1 GENERAL INFORMATION.............................................................4
1.1 Delivery Contents ..................................................................4
1.2 Manual Use............................................................................5
1.3 Device Description.................................................................5
1.4 Intended Use .........................................................................6
1.5 Symbols & Definitions ...........................................................6
2 WARNINGS & SAFETY REGULATIONS ..........................................7
2.1 Definitions .............................................................................8
2.2 Electrical Shock Hazards ........................................................8
2.3 Light Exposure Hazards .........................................................9
2.4 Other Hazards......................................................................11
3 HANDLING & OPERATION..........................................................12
3.1 Assembly Instructions .........................................................12
3.2 Handling...............................................................................13
3.3 Operation.............................................................................14
3.4 Advanced Options ...............................................................19
4 INDICATIONS..............................................................................21
4.1 UVB 311 nm – Indications ...................................................21
4.2 UVA (PUVA) – Indications....................................................21
5 CONTRAINDICATIONS................................................................22
6.1 UVB – Side Effects................................................................22
6.2 UVA – Side Effects ...............................................................23
7 TREATMENT RECOMMENDATIONS ...........................................26
7.1 UVB – Treatment Recommendations..................................27
7.2 UVA (PUVA) – Treatment Recommendations .....................29
8 TECHNICAL SPECIFICATIONS & INFORMATION .........................32
8.1 Technical Data .....................................................................32
8.2 Conditions for Transport, Storage & Operation ..................32
8.3 Cleaning & Disinfection .......................................................33

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 3 of 44
8.4 Electromagnetic Compatibility (EMC) ................................. 34
8.5 Spectral Range..................................................................... 37
8.6 Accuracy .............................................................................. 38
8.7 Maintenance & Repair ........................................................ 38
8.8 Lamp Ageing & Replacement .............................................. 38
9 OTHER INFORMATION............................................................... 39
9.1 Warranty ............................................................................. 39
9.2 Expected Service Life........................................................... 40
9.3 Disposal Note ...................................................................... 40
9.4 Equipment Classification ..................................................... 41
9.5 Contact Details .................................................................... 41
10 PATIENT REPORT ..................................................................... 42

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 4 of 44
1GENERAL INFORMATION
1.1 Delivery Contents
Item-No.
Description
Qty.
1004
OCTAderm UVA
1
The particular
device type can be
identified by the
corresponding
name plate on the
device.
1005
OCTAderm UVB 311 nm
80100
Patient goggles
2
User Manual
1
10092
Key
2
10115
Spacer
1
Optionally available accessories & spare parts
80100
Patient goggles
80200
Personnel safety goggles
83270
UVA tube 100 W
83221
UVB 311 nm tube 100 W
83269
Electronic starter

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 5 of 44
1.2 Manual Use
This user manual & technical description is an integral part of this
device. Anyone operating this device must read and understand
this manual in its entirety before operating the device, including
all warnings, cautions, and instructions.
Instructions vital to the safety of persons operating the device,
receiving treatment from the device and property, including but
not limited to the device, are contained in this manual. If these
instructions are not understood and followed, damage and
serious injury, including death, can be caused.
This manual conforms to all regulatory standards applicable to
the device at the time of manufacture of the device and the
original printing of the manual. All rights are reserved for the
device design and all associated materials, including software and
mechanical applications and methods, trade names and logos
used. The device and manual are subject to change without
notification. No part of this manual may be reproduced or used
for any purpose other than operating the device unless
expressed written consent is obtained from MEDlight GmbH.
1.3 Device Description
The OCTAderm from MEDlight is a medical full body
phototherapy device. A total of 8 UV light emitting lamps are
mounted in the front of the unit, which is accordingly marked
with the label OPTICAL RADIATION APERTURE. For accurate dose
application the device is equipped with a UV dosimetry which
measures the irradiance in real time, and corrects the exposure
time accordingly.
On the right side of the operating panel is a red push-button,
which is marked by a surrounding yellow ring with the label
EMERGENCY STOP. In an emergency, this button can be pressed to
turn off the UV light immediately.
USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 6 of 44
To the left of the emergency stop button is a key switch, with
which the device is turned on and off. To protect against
unauthorized use of the device, the key should always be
removed from the key switch when the device is not in use.
1.4 Intended Use
The device may only be used for such application areas, as
described in the manual. The device finds application in
dermatology, and is intended for the treatment of skin diseases
like psoriasis, atopic dermatitis, and vitiligo on all skin types. It
may only be operated by such persons who can ensure a proper
handling, due to their profession or their knowledge and practical
experience. The device may only be operated under permanent
supervision of the user.
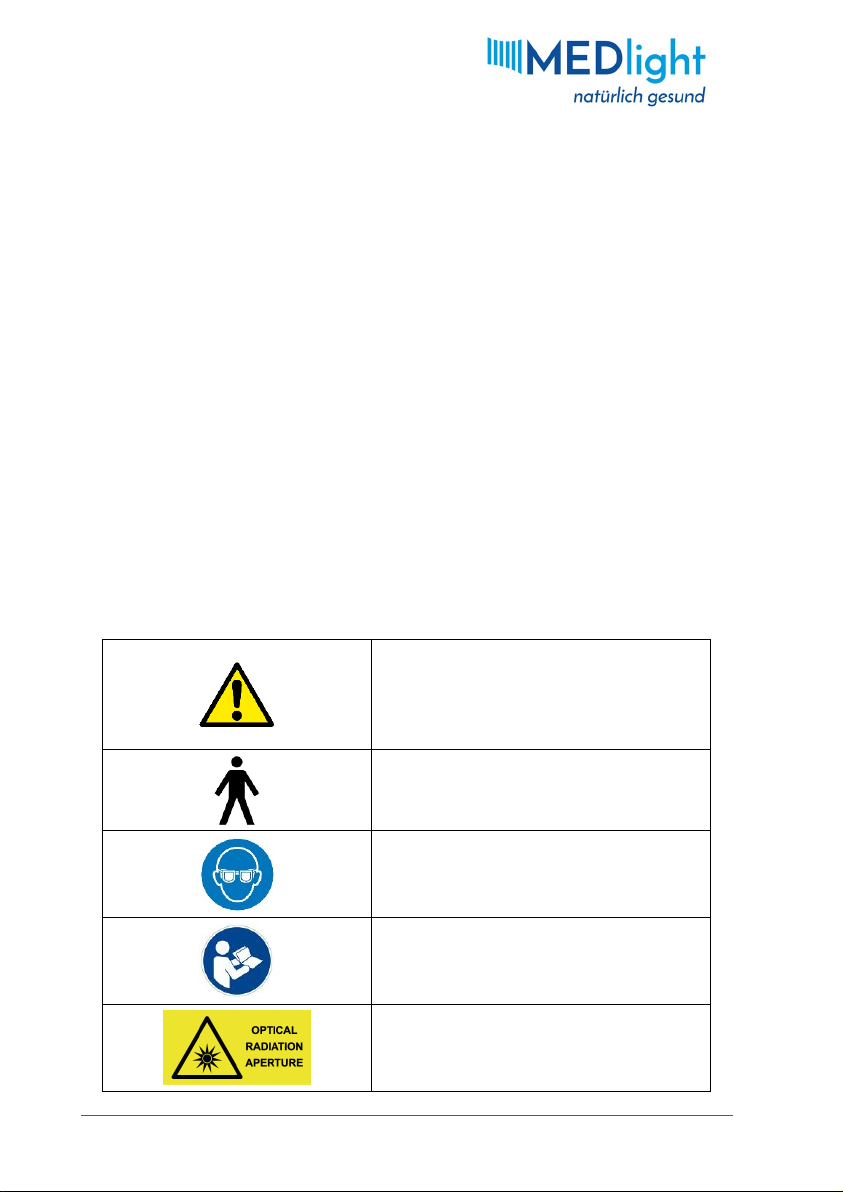
1.5 Symbols & Definitions
ATTENTION
Sections marked with
this symbol must be
read with special
attention
TYPE BEQUIPMENT
PROTECTIVE EYEWEAR MUST BE WORN
REFER TO USER MANUAL
OPTICAL RADIATION APERTURE

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 7 of 44
RISK GROUP 3
Device emits
potentially hazardous
UV light
HIGH VOLTAGE
MANUFACTURER
DATE OF MANUFACTURE
MASS OF MOBILE EQUIPMENT IN KILOGRAMS
SERIAL NUMBER
CATALOGUE NUMBER
DO NOT DISPOSE OF ELECTRICAL APPLIANCES
AS UNSORTED MUNICIPAL WASTE
2WARNINGS & SAFETY REGULATIONS
Note: Any serious incident that has occurred in relation to
the device should be reported to the manufacturer
and the competent authority of the Member State in
which the user and/or patient is established.

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 8 of 44
2.1 Definitions
Warning: Indicates a hazard. If not avoided, the hazard can
result in death or serious injury.
Caution: Indicates a potentially hazardous situation. If not
avoided, this hazard may result in minor personal
injury and/or product/property damage.
2.2 Electrical Shock Hazards
Warning: To avoid the risk of an electric shock, the
device must only be connected to a power supply
with a protective earth. The power supply must be in
accordance with all national and local installation
regulations. The device may only be operated with the
power supply voltage specified on the nameplate.
Warning: Prior to each use, always verify that the
device is in correct working order and operating
condition. Therefore check also plugs, wires, switches
and other control devices, as well as the irradiation
unit and all mechanical components. Upon discovery
of faulty, worn, or damaged component(s), MEDlight
authorized service personnel must replace the
component(s) and test the device prior to placing the
device in use again.
Warning: If you notice unusual noises, smoke, sparks
or burning smell from the device, immediately switch
it off and do not put it back in operation. Contact
MEDlight for a repair, and do not use the device again

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 9 of 44
until it is repaired, tested and released by MEDlight
authorized service personnel.
Warning: Open under no circumstances the device
housing. Turn off the device when it is not used.
Warning: Components marked with the HIGH VOLTAGE
symbol are connected to hazardous line voltages. In
single fault condition, conductive parts of these
components can carry dangerous line voltages.
Special care must therefore be taken during servicing
to avoid electric shock.
Warning: Place the device in such a way that the
mains plug or the mains connection remains easily
accessible at all times in order to enable a
disconnection from the mains.
Warning: Ensure that the device is serviced at least
annually, by MEDlight authorized service personnel.
2.3 Light Exposure Hazards
Warning: The UV light of the device can lead to
serious eye injury. To protect the eyes during
operation, patients must wear tightly fitting UV
blocking goggles, and the operator and anyone in
view of the device must wear protective UV blocking
glasses or goggles. Suitable UV blocking glasses and
goggles are included in the delivery contents. It is
recommended to provide an individual pair of glasses
for ever user, and an individual pair of goggles for
every patient. If required, further goggles can be
obtained from MEDlight. Furthermore it should be
avoided to look directly into the light, during

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 10 of 44
operation. In order to protect personnel from stray
UV in the vicinity of the device, it is recommended to
operate the device only in a designated treatment
room. Operating personnel exposed to UV radiation
for any prolonged periods of time should wear long-
sleeved clothing and gloves and use barrier creams
with a high light protection factor on exposed skin
areas.
Caution: To ensure an all-around homogenous light
exposure, the patient must stand in the middle of the
cabin. Instruct your patients to remain in this position
throughout the entire treatment to avoid partial
overdosing.
Caution: Use of controls or adjustment or
performance of procedures other than those specified
in this user manual may result in hazardous radiation
exposure.
2.3.1 Hazard Distance Information
The ocular hazard distance, respectively the skin
hazard distance is the distance within which the
maximum exposure level of the eye or skin is not
expected to result in adverse biological effects over
the course of a normal working day (8 hours). The
ocular hazard distance, respectively skin hazard
distance has been determined to be 10.33 m for the
UVA device and 14.85 for the UVB 311nm device.

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 11 of 44
2.4 Other Hazards
Warning: Modifications of the device are not allowed.
Operate the unit therefore only with original
equipment, supplied by MEDlight.
Warning: Do not store or operate the device in humid
areas. The device must never be directly exposed to
flowing or splashing liquid or water. Also, be careful
when cleaning with a damp cloth to ensure that no
liquid enters the device.
Warning: The device must not be operated in the
presence of a flammable aesthetic mixture with air,
oxygen or nitrous oxide or where flammable
substances such as alcohol, fuel or similar substances
are used.
Caution:Lay the power cord so that there is no
danger of tripping up a person.

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 12 of 44
3HANDLING & OPERATION
3.1 Assembly Instructions
Fig.1: Components of the unit

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 13 of 44
After the device ① has been unpacked, the castors-holders
②③④⑤ shown in Fig. 1 have to be inserted into the mounts
at the bottom of the unit. For this, each spring loaded button of a
castor-holder must be held down, in order for it to be inserted
into the socket until it snaps into the relevant hole in its socket in
the main unit. During assembly, pay attention to the two front
casters-holders which have a bush, into which the spacer ⑥has
to be inserted later on. The two front castors-holders must
therefore be mounted, so that the particular bush is facing
inwards. After the castors-holders are mounted, the spacer bar
can be inserted. To do so, the spacer bar will be first inserted into
one of the bushes, and must then be retracted into the opposite
direction, until the other end rests in the opposing bush.
3.2 Handling
Prior to the first use, a device training must be carried out by
MEDlight authorised personnel. Additional trainings can be
arranged upon request.
Plug the mains plug of the device in a protectively earthed outlet
which is fused with 16 amps fuses. As soon as the device is
switched on via the key switch in the operating panel it is ready
for use. Please, switch the device always off via the key switch, if
it is not in use for a longer time and at the end of each working
day.
The device should be protected against unauthorised use by
removal of the key from the key switch.
The electronic control of the device is located in the middle of
the operating panel, and allows a simple and intuitive operation.
The device is equipped with a dosimetry with UV sensor, which
continuously monitors the irradiance of the UV tubes during
treatments. This ensures a precise application of doses, even if
the irradiance of the UV tubes changes, because the exposure
times will be accordingly adjusted in real time by the dosimetry.

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 14 of 44
In an emergency, you can press the red push-button, which is
located on the right side of the operating panel and marked with
the label EMERGENCY STOP. This will immediately stop the
treatment and turn off all UV tubes. To reset the emergency stop
switch, turn the red actuation counter-clockwise until it springs
back.
3.3 Operation
Fig. 2: Controller
Fig. 3: Start button

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 15 of 44
3.3.1 Dose Input
Make sure that the emergency stop switch is unlocked (the green
ring below the red push button must be visible). Then switch on
the device using the key switch. The display shown in Fig. 4 now
appears on the device controller.
Fig. 4: Dose input
In case that the device shows e.g. "2 x"or "4 x"
instead of "1 x"below the word DOSE, you must read
section 3.4.2 AUTOMATIC THERAPY REPETITION first,
before you start a therapy!
With the + and - keys of the controller you can set a dose in
increments of 0.01 J/cm². The maximum dose for the OCTAderm
UVA (Item-No. 1004) is limited to 9.95 J/cm², and for the
OCTAderm UVB 311 nm (Item-No. 1005) the maximum dose is
limited to 3.00 J/cm².
The dose can be increased with the +KEY, and decreased with the
-KEY.
After the desired dose has been set, press the OK KEY to confirm.

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 16 of 44
3.3.2 Ready State
Fig. 5: Display before a treatment is started
The device is now ready for treatment, which is indicated by an
acoustic signal (two short beeps). Before the therapy is started,
check in this menu again the correctness of the previously set
dose. In case that a correction is required, press the ESC KEY to
return to the menu described in section 3.3.1.
The patient and everyone in sight of the device must now wear
tightly fitting UV goggles.
To maintain the correct exposure distance, the patient must
stand in front of the OCTAderm so that his feet reach the spacer.
To start the treatment, press the START button shown in Fig. 3,
which is located left of the controller. Immediately after that, the
UV tubes will be switched on automatically.
USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 17 of 44
3.3.3 Therapy
The beginning of the treatment and the associated activation of
the UV tubes is indicated by an acoustic signal (four short beeps).
Fig. 6: Display during the first seconds after therapy start
Like with normal fluorescent tubes, the UV tubes also need a few
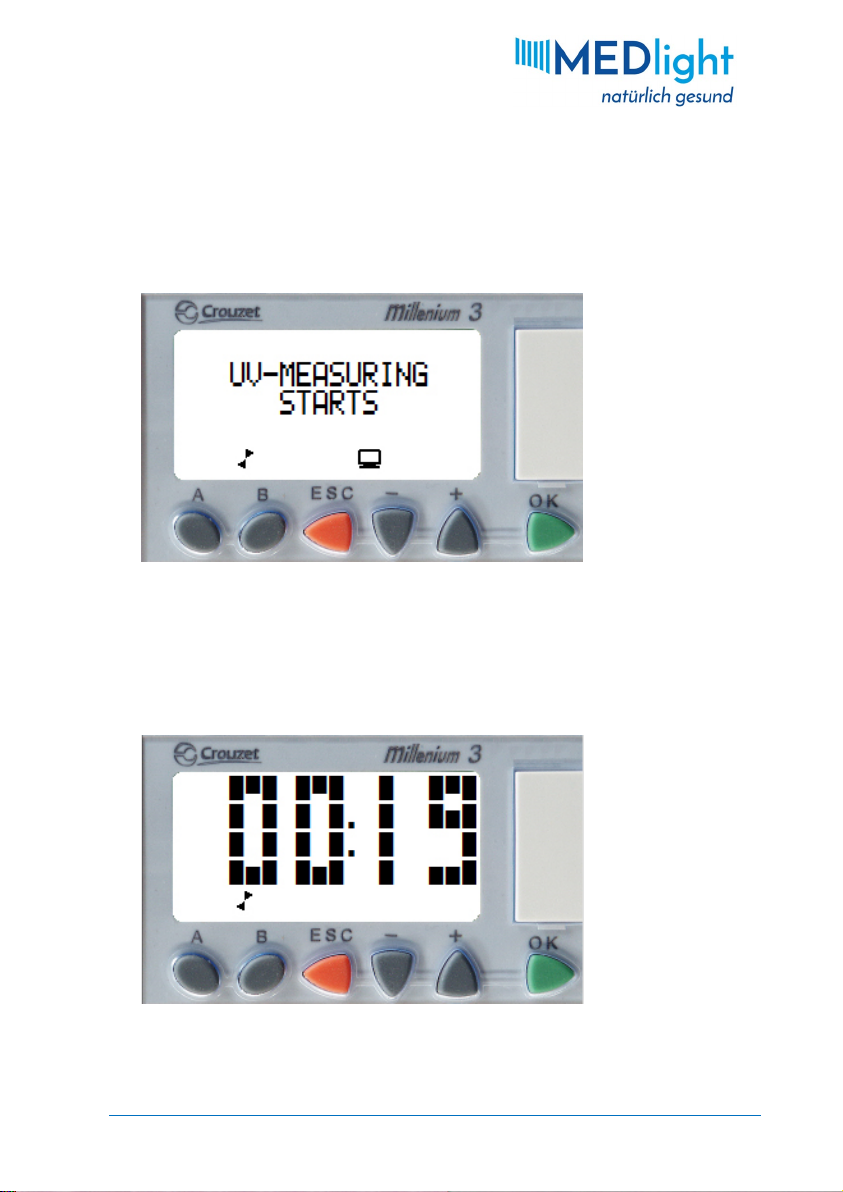
seconds for the ignition process. During this time the message
"UV-MEASURING STARTS" will be displayed, as shown in Fig. 6.
Fig. 7: Remaining time
Afterwards the remaining exposure time is displayed in minutes
and seconds (see Fig. 7). Since the OCTAderm measures the

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 18 of 44
emitted UV power with a sensor in real-time, any fluctuations in
the UV output will be considered in the calculation of the
exposure time. Such fluctuations in the UV intensity are also
reflected by the shown exposure time, which is therefore not
necessarily counting down in an exact second cycle.
When the end of the exposure time is reached, the UV tubes turn
off automatically and the end of the treatment is audibly
indicated. Now, the device can be switched off, by turning the
key switch to position 0.
In case that you need to abort a therapy, simply
press the ESC KEY of the controller or turn the key
switch into position 0.
3.3.4 Error Message
Fig. 8: Sensor fault
If the integrated sensor fails during therapy, the therapy will be
terminated, and the error message shown in Fig. 8 will appear on
the display of the controller. Please check first whether the
sensor may be obscured by dirt or other foreign bodies. The
sensor is mounted on an intermediate plate above the third tube
from the left. Close to the front of the operating panel is a hole of
about 5mm in diameter in the intermediate plate, which serves
USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 19 of 44
as measuring aperture. Make sure that this opening is not
obscured by dust, etc.
Then press the OK button of the controller and start a test
therapy. Do not forget to protect your eyes with UV goggles.
Check now if possibly some of the UV tubes have failed. If the
troubleshooting brings no result and the error still appears,
please contact the MEDlight customer service.
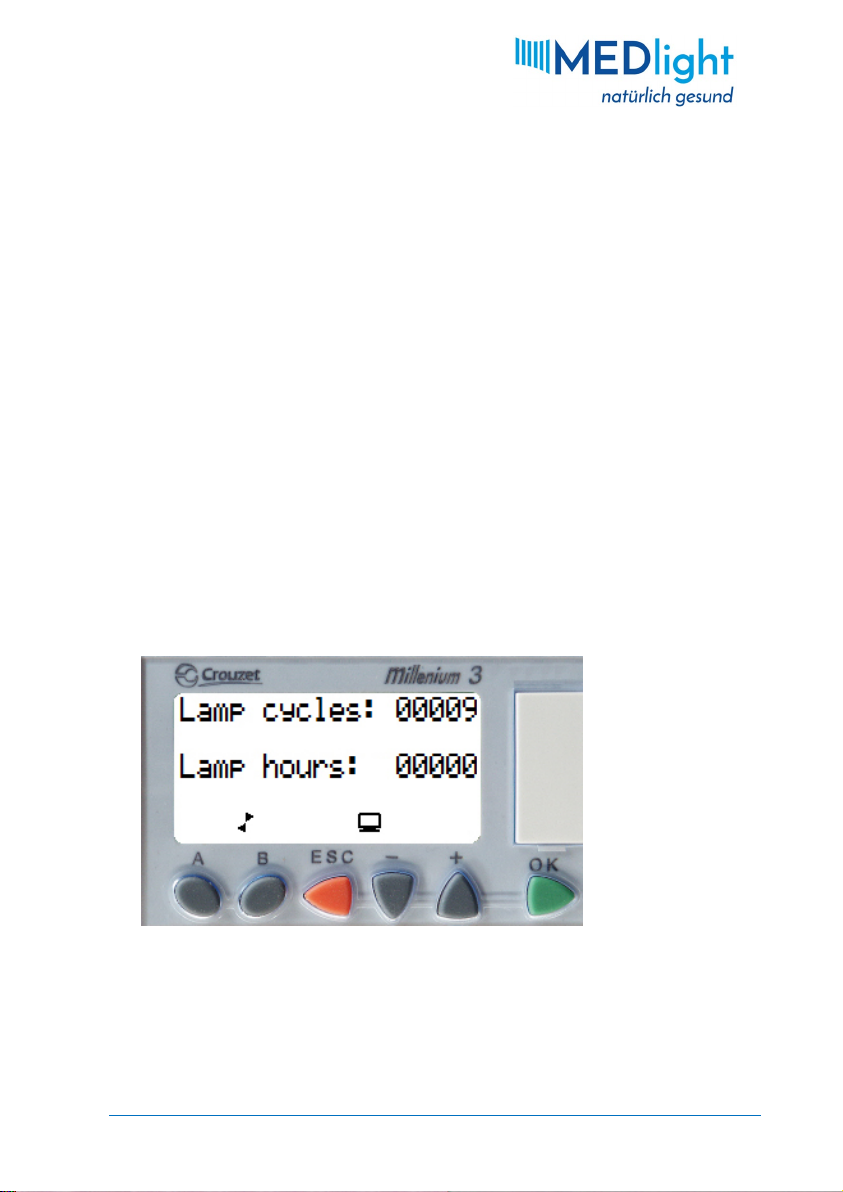
3.3.5 Operation Hours Display
The output of the UV tubes decreases with time, and also the
switching frequency affects the life of the tubes. For a
corresponding overview, the number of switching cycles as well
as the number of lamp hours can be displayed. Switch on the
device with the key switch, and then simultaneously press the A
KEY and BKEY of the controller. As long as both keys are held
down, the following display will be shown.
Fig. 9: Lamp cycles and operation hours
3.4 Advanced Options
The OCTAderm offers optional features which can be pre-set at
the factory according to customer preferences. These advanced
options can alter the previously described standard version.

USER MANUAL &
TECHNICAL DESCRIPTION
OCTAderm_User Manual_2020-01-10
Page 20 of 44
3.4.1 Dose Limit
By default, the OCTAderm UVB 311 nm (Item-No. 1005) is pre-set
to a maximum dose of 3.00 J/cm². If necessary, however, it is also
possible to change the standard factory setting. The dose limit
can be adjusted in increments of 0.05 J/cm² up to 9.95 J/cm².
3.4.2 Automatic Therapy Repetitions
For the OCTAderm, there are factory settings available for
optional therapy repetitions. The device can be preconfigured, so
that the chosen dose can be applied up to four times in a row.
This can for example be useful, if generally front and back will be
treated or if a treatment for all four sides is required. According
to the pre-set number of repetitions, the message "2 x" or "4 x"
appears on the display below the word DOSE, as shown in Fig. 4.
The therapy with pre-set therapy repetitions doesn't differ
significantly from the single therapy that is described in section
3.3. However, when the first exposure time is over, the tubes will
not switch off automatically. Instead, you will hear an audible
signal for a maximum of ten seconds.
Within this period, the body area next to be treated should be
exposed to the irradiation field. To confirm that the new
treatment position has been taken, the start button needs to be
pressed again.
If the START button is not being pressed within 10 seconds, the
UV tubes will switch automatically off for reasons of safety.
If the START button is pressed after the 10 seconds are over, the
tubes will switch on again and the therapy will continue. This
process is repeated until the pre-set number of therapy
repetitions has been achieved. After this, the end of the
treatment will audibly indicated and the tubes will switch off
automatically. Then you need to switch off the unit by turning
the key into position 0.
Table of contents
Other MEDlight Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Olympus
Olympus VISERA ELITE II Quick reference guide

Caldera
Caldera Benesta CAL-TR1511 Instructions for use

Micro-Pulse
Micro-Pulse I.C.E.S. M1 user manual

Playcore
Playcore SPECTRUM Aquatics Freedom Lift 57961 manual

Fisher Wallace
Fisher Wallace Stimulator instruction manual

orlvision
orlvision RSX-USB Instructions for use