IACER Srl 3 MNPG121
Compliance of the concerned product with the Directive 93/42/EEC
has been assessed and certified by the Notified Body
0476 - Kiwa Cermet Italia S.p.a.
Via di Cadriano 23 –40057 Cadriano di Granarolo (BO), Italia
Certificate no.: MED24021
following the certification procedure according to Annex II (excluding
point 4) of the Directive 93/42/EEC.
________________ _____________________
Place, date Signature
Classification
The I-TECH LA500 has the following classification:
•class IIa (Directive 93/42/EEC, Annex IX, rule 9, 10 and further
amendments);
•class I with B type applied part (classification EN 60601-1);
•class 3B laser (classification EN 60825-1);
•equipment unsuitable for use in presence of a flammable
anesthetic mixture containing air, oxygen and nitrous oxide;
•equipment suitable for continuous operation;
•equipment unsuitable for outdoors use.
Purpose and scope
Clinical intended use: Therapeutic
Environmental intended use: Ambulatory and in hospitals
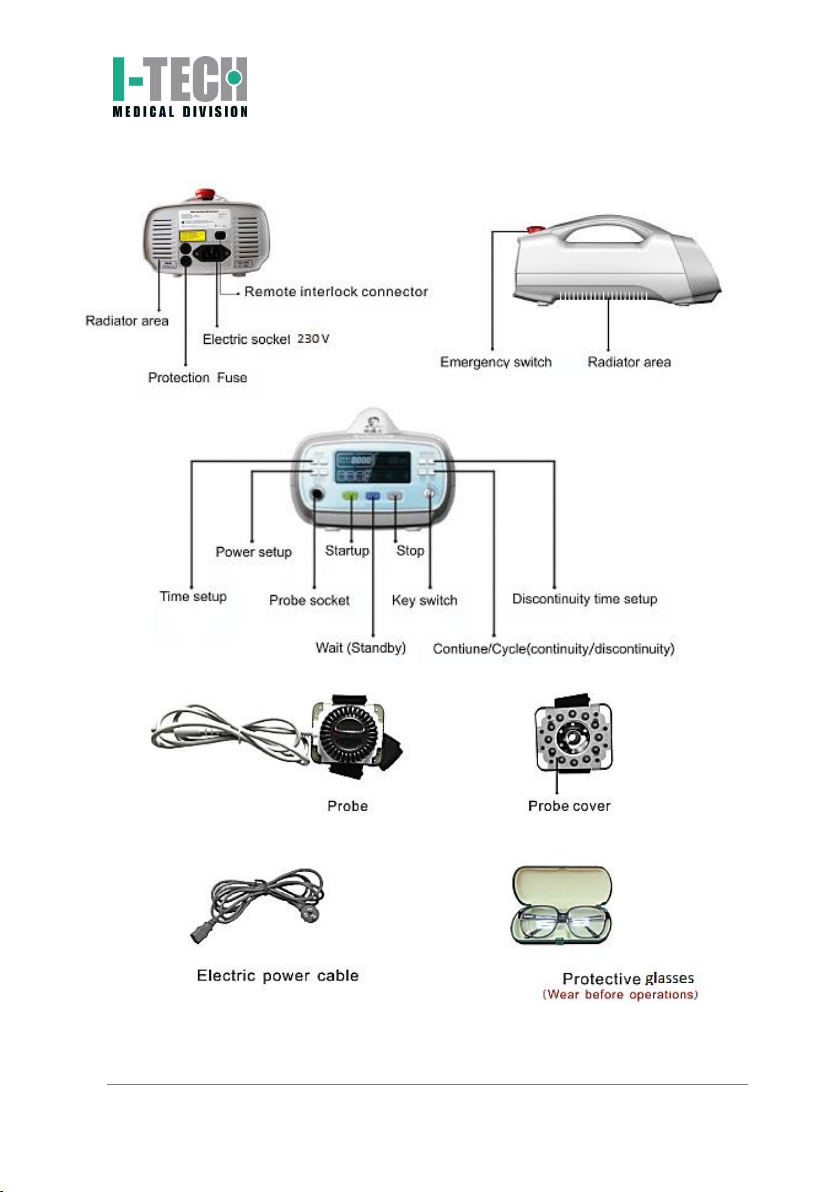
I-TECH LA500 is an electro-medical device that delivers treatments of laser-
therapy, with the help of power laser up to 500mW for the provision of
treatment though a specific probe.
I-TECH LA500 is an active therapeutic device, not invasive, used especially
by physiotherapists, physicians and pain therapists.
The use of I-TECH LA500 is indicated for professional user in clinics/hospitals.