neuroConn DC-Stimulator User manual

Programmable Direct Current Stimulator
DC-STIMULATOR
User manual




5
Table of contents
Table of contents
........................................................................................................................ 8
Preface
1
........................................................................................................................ 10
Safety
2
................................................................................................................ 11Important Notes
................................................................................................................ 14Safety aspects of transcranial Direct Current Stimulation (tDCS)
................................................................................................................ 15Safe stop mode
........................................................................................................................ 16
Getting Started
3
................................................................................................................ 16Components
.......................................................................................................... 17DC-STIMULATOR
.......................................................................................................... 20Equipment
.......................................................................................................... 20Consumables
................................................................................................................ 22Type label
................................................................................................................ 23Power supply
................................................................................................................ 25Activating the DC-STIMULATOR
................................................................................................................ 25Mode standby
................................................................................................................ 25Turning off the DC-STIMULATOR
........................................................................................................................ 27
Operating Basics
4
................................................................................................................ 28External charger
................................................................................................................ 29Electrodes
.......................................................................................................... 30Cleaning and storing
................................................................................................................ 31Impedance control
................................................................................................................ 33Additional hardware
.......................................................................................................... 33Trigger input
................................................................................................................ 34Cleaning the device
................................................................................................................ 35Moving the device
................................................................................................................ 37Storing the device
........................................................................................................................ 38
Software Reference Manual
5
................................................................................................................ 38Display, button, menu

Table of contents
6
................................................................................................................ 40PARAMETER
.......................................................................................................... 40tDCS
................................................................................................................ 41STIMULATION
................................................................................................................ 43SYSTEM
.......................................................................................................... 44Trigger input (optional)
.......................................................................................................... 45Impedance limit
.......................................................................................................... 46Load setting
.......................................................................................................... 47Study mode (optional)
....................................................................................................... 49Procedure
.......................................................................................................... 51Language set
.......................................................................................................... 52Signal tone
.......................................................................................................... 52Backlight brightness
................................................................................................................ 53MASTERCODE (optional)
........................................................................................................................ 55
Troubleshooting
6
........................................................................................................................ 56
Technical Specifications
7
........................................................................................................................ 58
Electromagnetic compatibility
8
................................................................................................................ 58Information for ensuring electromagnetic compatibility
................................................................................................................ 59Guidance and manufacturer's declaration - electromagnetic emissions
................................................................................................................ 59Guidance and manufacturer's declaration - electromagnetic immunity
................................................................................................................ 62
Recommended separation distances between portable and mobile RF
communication equipment and the device or system
........................................................................................................................ 64
Service
9
................................................................................................................ 65Safety inspection
........................................................................................................................ 66
Distributors
10
........................................................................................................................ 73
Intended use and therapeutic application
11
........................................................................................................................ 79
Disclaimer
12
........................................................................................................................ 80
Notes
13
........................................................................................................................ 81
Manuals Equipment
14
................................................................................................................ 81Rubber electrodes and sponge pads

Chapter 1 - Preface
8
Preface1
Thank you for buying a product of neuroConn GmbH.
The DC-STIMULATOR allows you to perform a transcranial direct current stimulation
(tDCS) as part of a non-invasive Interventional Neurophysiology. Weak electrical currents
(max 2 mA) at a duration of 15..30 minutes are applied at different positions on the head.
The electrical charge and current density applied during tDCS are far below the threshold for
releasing a stimulus and take modular effect to existing neuronal elements.
Depending on duration, used current, current density and frequency the stimulation is
effective on inhibiting or activating cortical activity.
If established treatments fail a supporting therapeutic effect on depression and neuropathic
pain in the lower limbs is probable and during rehabilitation of speech and motor skills after
stroke and on auditory hallucinations in schizophrenia possible.
Users of the DC-STIMULATOR are physicians and psychologists with knowledge about the
effects of non-invasive brain stimulation and experience in brain stimulation.
The essential performance of the DC-STIMULATOR during cranial electrotherapy is to
ensure that:
•a maximum output current of 2 mA will not be exceeded (max. tolerance 5 %)
•a predefined stimulation time will not be exceeded (max. tolerance (1%)
This manual shows you how to operate the DC-STIMULATOR.
The devices of the neuroConn GmbH are delivered with user manuals in English or German
language (Germany, Austria and Switzerland) depending on the destination country.
The manual contains all the information required by Directive 93/42/EEC Annex I Section 13.
Also the standards EN1041:2008 (Providing of information by the manufacturer of medical
devices) and EN980:2008 (Symbols for the labeling of medical products) as well as
EN60601:2006 (Medical electrical equipment; part 1: general requirements for safety)
including the essential performance characteristics: table D.1 – Common symbols & table
D.2 safety marks are applied.

Chapter 1 - Preface
9
Note
The following signs bring important information to your attention:
This informs the user that failure to follow these instructions may cause harm to
the user and others or may damage the DC-STIMULATOR or other equipment.
This is a general hint or useful advice for better use of the DC-STIMULATOR.

Chapter 2 - Safety
10
Safety2
The DC-STIMULATOR has been certified as an active medical device class IIa.
CAUTION FOR UNITED STATES OWNERS AND OPERATORS:
Investigational Device. Federal (or US) law limits this device to
investigational use.
The construction of the DC-STIMULATOR conforms to the regulations set out in the
Medical Device Directive 93/42/EEC (Date of issue 14th June 1993), which was put into
German law. The requirements of the following standard(s) or normative document(s) are
fulfilled:
-
EN 60601-1:2006 Medical electrical equipment Part 1: General requirements for safety
-
EN 60601-1-2:2007 Medical electrical equipment Part 1-2: General requirements for
safety - Collateral standard: Electromagnetic compatibility - Requirements and tests
-
EN 62304:2006 Medical device software - Software life-cycle process
-
EN 62366:2008 Medical devices - Application of usability engineering to medical devices
-
EN ISO 10993-1:2009 Biological evaluation of medical devices - Part 1: Evaluation and
testing within a risk management process (ISO 10993-1:2009)
-
EN ISO 14971:2012 Medical devices - Application of risk management to medical devices

Chapter 2 - Safety
11
Important Notes
Stimulation currents of greater than 2,000 µA or stimulation durations of
longer than 20 min are for research purposes only. The manufacturer
assumes no liability for any injury in these cases.
DC currents can harm body tissue. Ensure that limitations for current density are
adhered to. The German authority ”Bundesinstitut für Arzneimittel und
Medizinprodukte (BfArM)“ recommends a current density limit of 0.1 mA/cm². The
manufacturer assumes no responsibility for any injury caused by a too high
current density.
Modifications and repair of the DC-STIMULATOR must be carried out only by the
manufacturer or a company authorized by the manufacturer.
The DC-STIMULATOR must never be opened. The manufacturer assumes no
responsibility for any damage caused by such a practice. If any technical
problems are experienced, always inform the dealer or manufacturer.
Medical electrical devices such as the DC-STIMULATOR are subject to
particular precautions regarding EMC and must be installed and operated
according to established practice.
Portable and mobile HF communication equipment can influence electrical
devices such as the DC-STIMULATOR.
The DC-STIMULATOR is not protected against liquid spills (IEC 60529: IP20).
The operator should avoid handling liquids when using the device as there is a
risk of electric shock. Should liquid spill onto the device, please unplug the device
and inform the dealer or manufacturer immediately.
The device must not get in contact with liquid spills because liquid spills can
damage the device and can be a risk for the patient, the operator or third persons.
Chapter 2 - Safety
12
The DC-STIMULATOR must not be used in combination with a defibrillator as it
has no appropriate protection. The manufacturer accepts no responsibility for any
injury caused by such use.
The DC-STIMULATOR must not be used on patients with a pacemaker or brain
stimulator as such use can interfere or damage these devices. The manufacturer
accepts no responsibility for any injury caused by such use.
The DC-STIMULATOR must not be used on patients with implanted intracranial
metals such as clippings, coilings, ventriculo-peritoneal shunts, endoprosthesis
etc.. The manufacturer accepts no responsibility for any injury caused by such
use.
For safety reasons, never use bipolar stimulation on any other part of the
body apart from the head. Bipolar stimulation setups can harm the heart
should the electrodes be misplaced. The manufacturer accepts no
responsibility for any injury caused by such use.
There is the possibility of an electrostatic discharge by touching the patient (for
example the patient's head) or the DC-STIMULATOR. Having an electrostatic
discharge while electrodes are attached to a subject may cause a discharge
current to flow through the electrodes leading to a shock sensation similar to that
experienced in everyday life. Such currents are not dangerous but they are
unpleasant. Please avoid touching the patient during stimulation.
The patient must not touch the contacts at the rear side of the device.
NB: the human body reacts differently to direct current (DC) stimulation.
Chapter 2 - Safety
13
The output circuit of the constant current source of the DC-STIMULATOR is
equipped with an electrical fuse which limits the current to 5 mA. Therefore, in any
faulty condition and during normal operation, the fuse will become open circuit if
the current exceeds 5 mA by a significant amount. The higher the current
exceeds 5 mA that faster it will become open circuit.
Do not disconnect the electrodes if current is flowing as this will cause a strong
stimulus to be delivered.
The DC-STIMULATOR must not be positioned or run close to flammable
mixtures of anesthetic gas because it has no appropriate protection.
The DC-STIMULATOR must not be positioned or run in an oxygen rich
environment because it has no protection against a risk of fire.
You must not connect any devices / components / cables, that are not described
in this manual or that are not part of the delivery, to the inputs or outputs of the
system.
During the use of the DC-STIMULATOR on patients interventions on the device
must not be performed.
A damaged DC-STIMULATOR must not be connected to the patient.
Damaged devices or accessory components must not be connected to the DC-
STIMULATOR.
With opened skull or after trepanation a stimulation with the DC-STIMULATOR
must not be performed.
Chapter 2 - Safety
14
Safety aspects of transcranial Direct Current Stimulation (tDCS)
Attention! - In following situations the patient might be injured!
Only place the electrodes on healthy skin. If there are known allergies you should
consult a general practitioner or dermatologist first. Never use it with injured skin
areas. Stimulation of injured skin areas might result in redness of the skin
(erythema) and skin burns. The manufacturer accepts no responsibility for any
injury caused by such use.
Never use tap water to wet the sponges or the skin before or during the
stimulation. This might result in skin burns! Always use 0,9 % NaCl solution!
The manufacturer accepts no responsibility for any injury caused by such use.
If you attach the electrodes with electrode paste only use the electrode paste
supplied by the manufacturer. The use of other electrode pastes and gels might
result in in redness of the skin (erythema) and skin burns. The manufacturer
accepts no responsibility for any injury caused by such use.
Thermal limit for current density
To avoid burning the patient, the German authority (Bundesanstalt für Arzneimittel und
Medizinprodukte) gives a limit of 0.1 mA/cm²for DC current applications. Observations of
tDCS stimulated patients show, that even current densities as low as 0.028 mA/cm²can
sometimes be painful.
E.g.: Using electrodes with a surface area of 35 cm²with a current of 1 mA applies a
current density of 0.02857 mA/cm².
Histological limit for current density
To avoid permanent injury of tissue, current density should not be higher than 25 mA/cm².
This limit is far above the limit for thermal effects of the current density.
Chapter 2 - Safety
15
Histological limit for the duration of DC current applications
To avoid permanent injury of tissue, duration of DC current applications should be
temporary. The charge per surface area should not exceed a value of 216 C/cm².
E.g.: Using electrodes with a surface area of 35 cm²with a current of 1 mA over a
period of 15 min applies a charge of 0.025 C/cm².
To calculate the charge per surface area for intermittent DC current, the current density
must be included as well as the number of pulses and its duration.
Safe stop mode
At high currents, aborting the stimulation causes an unpleasant, sometimes even painful
“current leap” for the patient. The “safe stop mode” can prevent this by reducing the current
slowly and continuously (1 mA per second) down to 0 µA.
The “safe stop mode” is active in any stimulation mode and works during either manual and
automatic abortion since it exceeds thresholds of impedance, current or voltage, as well as
during the turning on and off the device.
The output current is continuously monitored by the microcontroller program, but
it needs a finite time to discover high impedances and start the “safe stop mode”
procedure. If there is a very short lasting interruption of the current path that lasts
in the order of 200-500 ms or less the stimulator will not detect it. Short
interruption of the current path has to be avoided during direct current stimulation.
Do not disconnect the electrodes if current is flowing for this will cause a strong
stimulus to be delivered.

Chapter 3 - Getting Started
16
Getting Started3
General conditions
Before using the DC-STIMULATOR, please read the following advice to make sure a proper
environment is provided:
-
The room temperature should be between 10 and 40 °C (50 and 104 °F) and the air
humidity should be between 20 and 93 % (non condensing). The air pressure must be
between 690 and 1080 hPa (3000 m).
-
Keep the system away from direct sunlight, heat sources, liquids or corrosive chemicals.
-
Keep the system away from magnetic objects. It can be damaged by too strong magnetic
fields.
-
Keep the system away from strong electric or electromagnetic fields.
If the device has been exposed to low temperatures or to drastic temperature
fluctuation (e.g. during transport), the resulting condensation might damage the
device. For safety reasons, wait until the DC-STIMULATOR has reached room
temperature (at least 1 hour) before using the device. The manufacturer accepts
no responsibility for any injury caused by insufficient acclimatization of the device.
The DC-STIMULATOR is suitable for mobile use and can be carried carefully around within
the range of its attached cables, even whilst in operation.
Components
The following instructions refer to the DC-STIMULATOR equipped with all
options. Depending on the specification of your DC-STIMULATOR some of the
sockets / components might not be available on the system.
You must not connect any devices / components / cables, that are not described
in this manual or that are not part of the delivery, to the inputs or outputs of the
system.

Chapter 3 - Getting Started
17
DC-STIMULATOR
DC-STIMULATOR upside
Figure 1: Upside of DC-STIMULATOR
DC-STIMULATOR bottom side
Figure 2: Bottom side of the DC-STIMULATOR
Chapter 3 - Getting Started
18
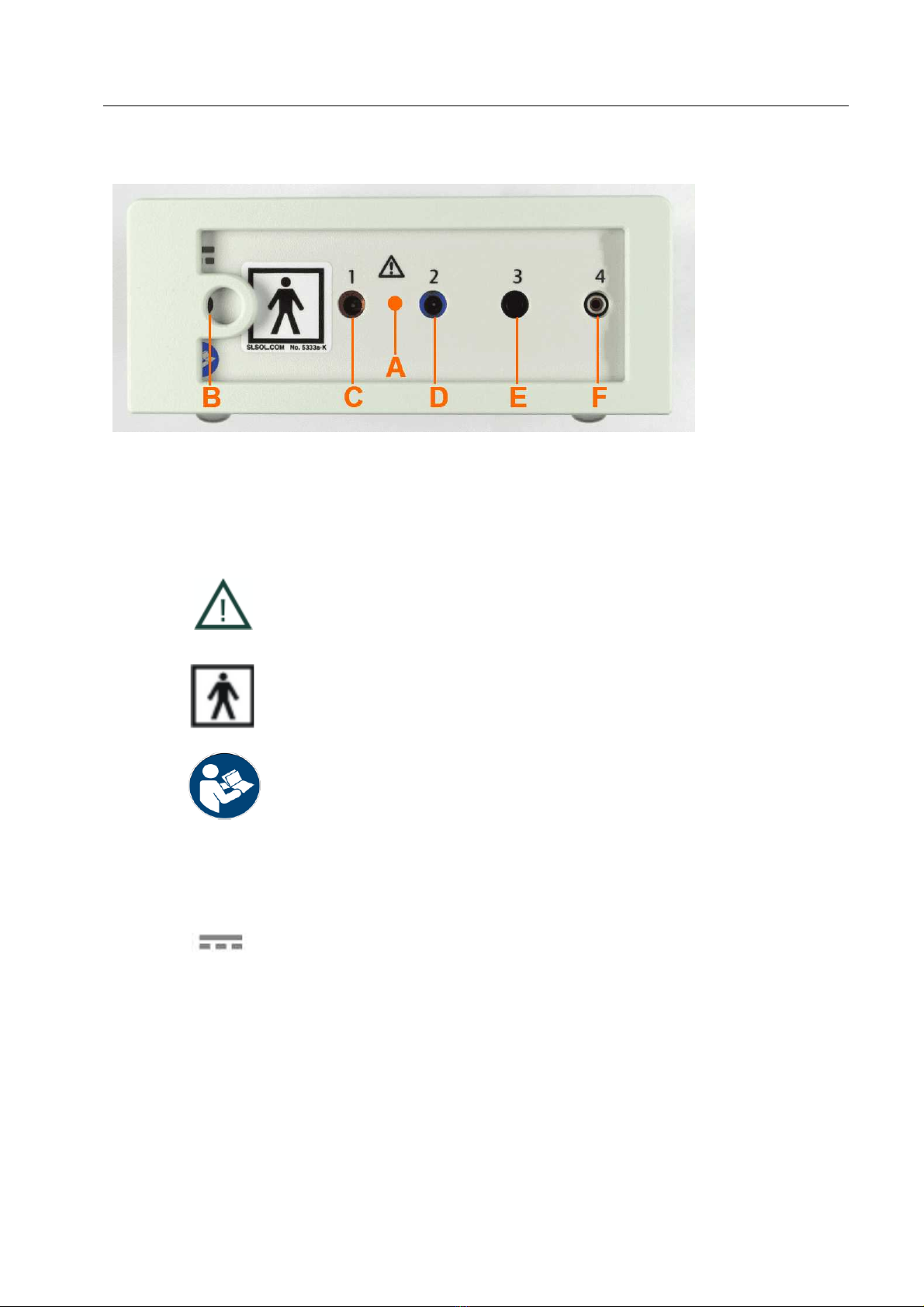
DC-STIMULATOR front side
Figure 3: Front side of the DC-STIMULATOR
Label/
icon
Specifications
Pay attention! It is necessary to note the manual's instructions for
this socket!
applied part BF
refer to instruction manual (ISO 7010-M0002)
A
moveable front plate
B
socket for charger
C
1
socket "1" (anode - positive) for touch-proofed safety connectors
according to DIN 42802-2 (ø 1.5 mm)
D
2
socket "2" (cathode - negative) for touch-proofed safety
connectors according to DIN 42802-2 (ø 1.5 mm)

Chapter 3 - Getting Started
19
E
3
socket "3" (ground) for touch-proofed safety connectors ø 2 mm
to connect the adapter box TRIGGER IN
F
4
socket "4" (signal) for touch-proofed safety connectors ø 2 mm to
connect the adapter box TRIGGER IN
DC-STIMULATOR rear side
Figure 4: Rear side of the DC-STIMULATOR
The patient must not touch the contacts at the rear side of the device.

Chapter 3 - Getting Started
20
Equipment
Adapter box TRIGGER IN
This adapter box is a module for connecting external trigger sources to the DC-
STIMULATOR. It consists of the following components:
Figure 5: Adapter box TRIGGER IN
1
adapter box
2
BNC socket
3
touch-protected connecting cable ø 2 mm
to plug in into socket 4 (signal) of the DC-
STIMULATOR
4
touch-protected connecting cable ø 2 mm
to plug in into socket 3 (ground) of the DC-
STIMULATOR
Consumables
Starter set
The rubber electrodes und sponge pads of the starter set are available in different sizes. The
standard starter set contains rubber electrodes 5 x 7 cm (35 cm2) and the appendant
sponge pads.
Table of contents