NINGBO DAVID YP-970 User manual

EDITION/REVISION B/1
DAVID RNINGBO DAVID
OPERATOR’S MANUAL
YP-970
INFANT INCUBATOR Please take well care of it.

NINGBO DAVID MEDICAL DEVICE CO., LTD.
ADD: NO.158 DAQING ROAD, SHIPU, NINGBO, ZHEJIANG PROVINCE, CHINA
POST CODE: 315731 FAX: 0086-574-65962111
MARKETING CENTER: NO. 100, JINGHUA ROAD, HI-TECH INDUSTRIAL DEVELOPMENT ZONE,
NINGBO, CHINA
POST CODE: 315000
TEL: 0086-574-87800008, 87800009 FAX: 0086-574-87801111
WEBSITE: http://www.nbdavid.com
EUROPEAN REPRESENTATIVE: SHANGHAI INTERNATIONALHOLDINGCORP. GMBH(EUROPE)
ADD: EIFFESTRASSE 80, 20537 HAMBURG, GERMANY
TEL: 0049-40-2513175 FAX: 0049-40-255726
SPECIAL STATEMENT: All of the content in the manual is checked carefully, if there is
any error or content of printing misunderstanding, our company
retains finally explanation of this card-usage.
NOTE: The product’s appearances maybe differ from the one in this manual, but it dose
not affect the capability of product. Please understand if it brings you troubles.

EDITION/REVISION B/2
Guidance and manufacture’s declaration – electromagnetic emissions-
for all EQUIPMENT and SYSTEMS
Guidance and manufacture’s declaration – electromagnetic emission
The YP-970 Infant Incubator is intended for use in the electromagnetic environment specified below. The customer of
the user of the YP-970 Infant Incubator should assure that it is used in such and environment.
Emission test Compliance Electromagnetic environment – guidance
RF emissions
CISPR 11 Group 1
The YP-970 Infant Incubator uses RF energy only
for its internal function. Therefore, its RF
emissions are very low and are not likely to cause
any interference in nearby electronic equipment.
RF emission
CISPR 11 Class B
Harmonic emissions
IEC 61000-3-2 Class A
Voltage fluctuations/
flicker emissions
IEC 61000-3-3 Complies
The YP-970 Infant Incubator is suitable for use in all
establishments, including domestic establishments and
those directly connected to the public low-voltage
power supply network that supplies buildings used for
domestic purposes.
Page 1of 4

Guidance and manufacture’s declaration – electromagnetic immunity –
for all EQUIPMENT and SYSTEMS
Guidance and manufacture’s declaration – electromagnetic immunity
The YP-970 Infant Incubator is intended for use in the electromagnetic environment specified below. The customer or
the user of YP-970 Infant Incubator should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment
- guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air Floors should be wood, concrete
or ceramic tile. If floor are
covered with synthetic material,
the relative humidity should be
at least 30%.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV for power supply
lines
±2kV for power
supply lines
Mains power quality should be
that of a typical commercial or
hospital environment.
Surge
IEC 61000-4-5
±1 kV differential mode
±2 kV common mode
±1 kV differential
mode
±2 kV common mode
Mains power quality should be
that of a typical commercial or
hospital environment.
Voltage dips, short
interruptions and
voltage variations on
power supply input
lines
IEC 61000-4-11
<5% UT
(>95% dip in UT)
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
(>95% dip in UT)
for 5 sec
<5% UT
(>95% dip in UT)
for 0.5 cycle
40% UT
(60% dip in UT)
for 5 cycles
70% UT
(30% dip in UT)
for 25 cycles
<5% UT
(>95% dip in UT)
for 5 sec
Mains power quality should be
that of a typical commercial or
hospital environment. If the user
of the YP-970 Infant Incubator
requires continued operation
during power mains
interruptions, it is recommended
that the YP-970 Infant Incubator
be powered from an
uninterruptible power supply or
a battery.
NOTE U
Tis the a.c. mains voltage prior to application of the test level.
Page 2of 4
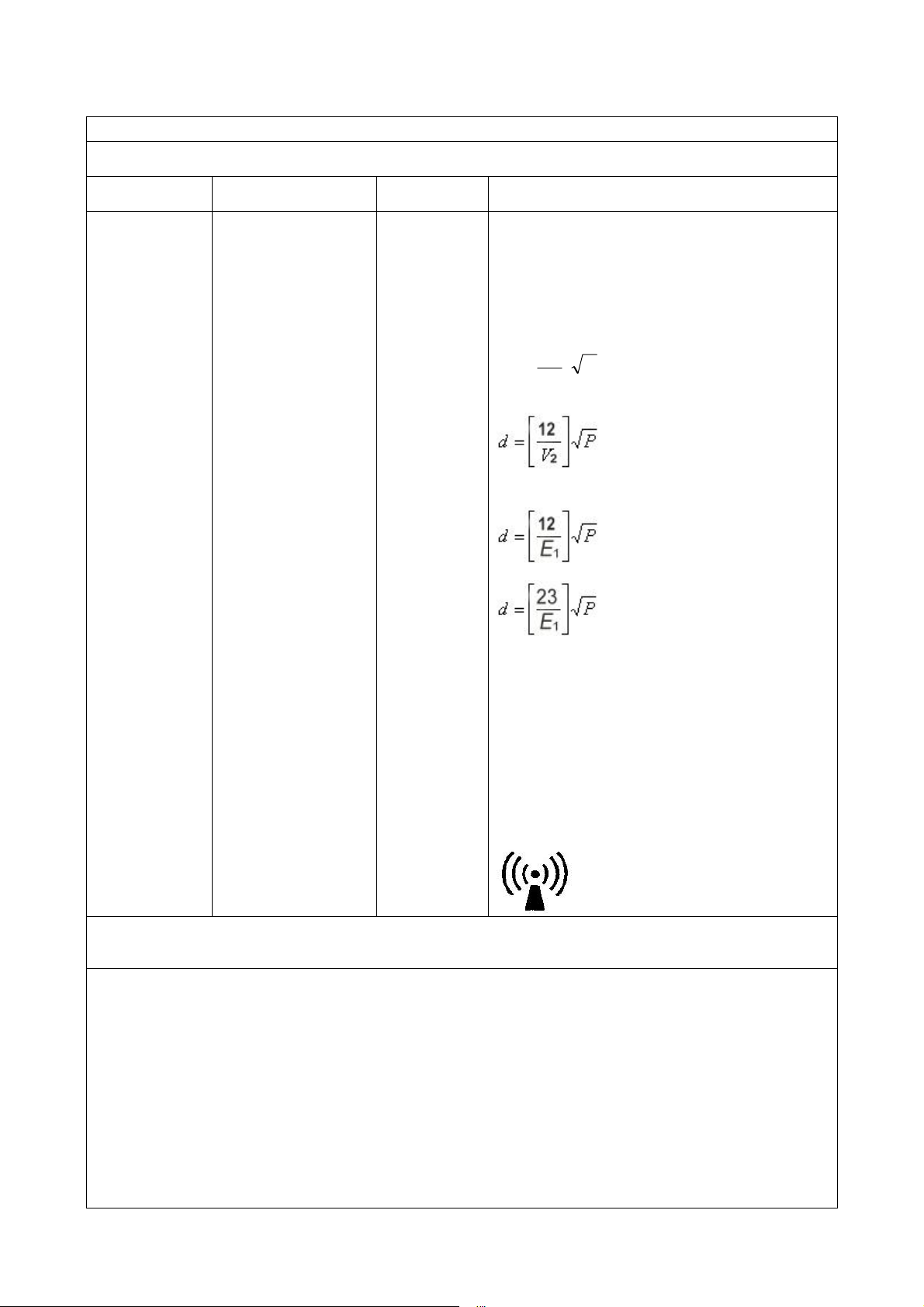
Guidance and manufacture’s declaration – electromagnetic immunity –
for EQUIPMENT and SYSTEMS that are LIFE-SUPPORTING
Guidance and manufacture’s declaration – electromagnetic immunity
The YP-970 Infant Incubator is intended for use in the electromagnetic environment specified below. The customer or
the user of YP-970 Infant Incubator should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance
level Electromagnetic environment - guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3Vrms
150 kHz to 80 MHz
outside ISM bandsa
10 Vrms
150 kHz to 80 MHz
in ISM bands
10 V/m
80 MHz to 2.5 GHz
3 Vrms
10 Vrms
10 V/m
Portable and mobile RF communications equipment
should be used no closer to any part of the YP-970
Infant Incubator, including cables, than the
recommended separation distance calculated from
the equation applicable to the frequency of the
transmitter.
Recommended separation distance
P
V
d⎥
⎦
⎤
⎢
⎣
⎡
=
1
5.3
80 MHz to 800 MHz
800 MHz to 2.5 GHz
Where Pis the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and dis the recommended separation
distance in metres (m).b
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey,c
should be less than the compliance level in each
frequency range.d
Interference may occur in the vicinity of equipment
marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
a The ISM(industrial, scientific and medical) bands between 150kHz and 80MHz are 6.765MHz to 6.795MHz; 13.553
MHz to 14.567 MHz; 26.957 MHz to 27.283 MHz; and 40.66 MHz to 40.70 MHz.
b The compliance levels in the ISM frequency bands between 150kHz and 80MHz and in the frequency range 80 MHz
to 2.5GHz are intended to decrease the likelihood that mobile/portable communications equipment could cause
interference if it is inadvertently brought into patient areas. For this reason, an additional factor of 10/3 is used in
calculating the recommended separation distance for transmitters in these frequency ranges.
cField strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with
accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey
should be considered. If the measured field strength in the location in which the YP-970 Infant Incubator is used
exceeds the applicable RF compliance level above, the YP-970 Infant Incubator should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or
relocating the YP-970 Infant Incubator.
dOver the frequency range 150 kHz to 80 MHz, field strengths should be less than 10 V/m.
Page 3of 4
Recommended separation distances between portable and mobile
RF communications equipment and the EQUIPMENT or SYSTEM –
for EQUIPMENT or SYSTEM that are LIFE-SUPPORTING
Recommended separation distances between
portable and mobile RF communications equipment and the YP-970 Infant Incubator
The YP-970 Infant Incubator is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the YP-970 Infant Incubator can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications
equipment (transmitters) and the YP-970 Infant Incubator as recommended below, according to the maximum output
power of the communications equipment.
Separation distance according to frequency of transmitter
(m)
Rated
maximum
output power
of transmitter
(W)
150 kHz to 80 MHz
outside ISM bands
P
V
d⎥
⎦
⎤
⎢
⎣
⎡
=
1
5.3
150 kHz to 80 MHz
in ISM bands
80 MHz to 800
MHz
800 MHz to 2.5 GHz
0.01 0.1167 0.12 0.12 0.23
0.1 0.3689 0.3795 0.3795 0.7273
1 1.1667 1.2 1.2 2.3
10 3.6893 3.7947 3.7947 7.2732
100 11.6667 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in
metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 The ISM(industrial, scientific and medical) bands between 150kHz and 80MHz are 6.765MHz to
6.795MHz; 13.553 MHz to 14.567 MHz; 26.957 MHz to 27.283 MHz; and 40.66 MHz to 40.70 MHz.
NoTE 3 An additional factor of 10/3 is used in calculating the recommended separation distance for transmitters in
the ISM frequency bands between 150kHz and 80 MHz and in the frequency range 80 MHz to 2.5 GHz to decrease
the likelihood that mobile/portable communications equipment could couse interference if it is inadvertently brought
into patient areas.
NOTE 4 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption
and reflection from structures, objects and people.
Page 4 of 4

i
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/0
WARRANTY
The product being described in this manual is warranted against defects in materials or
workmanship for one year from the date of shipment, with the following exceptions.
1. All consumable and disposable products are guaranteed to be free from defects upon
shipment only.
2. Calibrations are considered normal maintenance and are not included in the 1-year
warranty.
During the warranty period any defective parts other than those listed above will be replaced
at no charge to the customer.
This warranty is rendered void and our company cannot be held liable for conditions resultant
therefrom if:
1. Damage to the unit is incurred as a result of mishandling.
2. The customer fails to maintain the unit in a proper manner.
3. The customer uses any parts, accessories, or fittings not specified or sold by our company.
4. Sale or service is performed by the non-certified service/dealer agency.
This warranty is in lieu of all other warranties, expressed or implied, and our company shall in
no event be liable for incidental or consequential damages including loss of use, property damage,
or personal injury resulting from breach of warranty.
The Accreditation Manual for Hospitals requires each piece of equipment to be tested prior to
initial use and at least annually thereafter. To comply with this standard, we recommend that you
participate in our accreditation Testing compliance Program during the warranty period. This
service can be performed through our company and authorized dealers.
SERVICE
For optimal performance, product service should be performed only by authorized and
qualified service personnel. Please contact the local agency or the After-Sales of our company to
get more technical information about maintenance.

ii
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/1
OPERATING PRECAUTIONS
1. INFANT INCUBATOR (incubator) belongs to high risk medical device which can endanger
infant's life. Therefore please use the device only in neonate nursing room, children nursing
room, pediatric intensive care unit or similar sickroom in hospital. Operator for the device should
be special trained and operate the device under the instruction of medical practitioner.
2. The operator must keep observing the patient's condition while the device is working. Supervise
and record baby's temperature regularly to check whether the temperature of the patient is over
high/low or any other unusual conditions happen.
3. Please stop using the device when it failure or disfunction. Turn off the power and tansfer the
patient out from the device, then inform our company or our authorized agency for service. DO
NOT ask for service from person who's not been authorized by our company.
4. Direct radiation from sunlight or other infrared source could cause overheating of the infant
without activating the Over Temperature Alarm. DO NOT leave the INCUBATOR in direct
sunlight or near other sources of radiant heat.
5. DO NOT leave the INCUBATOR in the presence of flammable anaesthetic gases or other
flammable materials, such as some types of cleaning fluids.
6. DO NOT leave the INCUBATOR in the presence of strong electromagnetic field.
7. Devices which are easily interfered by magnetic field should not be used near the INCUBATOR
because they may interfered by the INCUBATOR.
8. The incubator does not equip the air cleaner, to make sure the good air quality inside hood, the
incubator should be used in the environment with clean air.
9. Please DO NOT use the INCUBATOR under working environment not stipulated in table 1.1, or
else, it may cause the failure or the INCUBATOR can not reach the requirements.
10. To prevent harm to the infant, the hood should not be raised while leads are connected to the
infant or if the mattress tilted.
11. There should be no need to raise the hood at any time while the infant is cared for in the
incubator. All necessary access to the infant can be achieved by means of the Access Panel
and Access Doors.
12. When the Access Panel is open, the temperature from the air temperature indicator maybe not
the real temperature inside incubator. Therefore, do not leave the Access Panel open much
longer.
13. All access panel‘s latch should be firm plugged, in case accidental open.
14. For infant safety, DO NOT leave the infant unattended when the Access Panel is open.
15. Other accessories within the incubator which can alter the air flow pattern may affect
temperature uniformity and temperature variability.
16. Turning on Phototherapy unit may affect hood wall temperature, incubator air temperature and
infant skin temperature, at this time, we suggest the incubator should be worked in the baby
mode, otherwise the set air temperature of the thermotherapy devices has to be reduced
according to body temperature measurement.

iii
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/1
17. Patient safety and incubator performance may be compromised if air flow passages are not
kept clear of obstructions (blankets, stuffed animals, etc.) during clinical usage.
18. Do not place surgical covers or blankets over the infant and warm air curtain or side vents
simultaneously. This may cause heat induced injury and burns.
19. Removing the incubator should be finished at least by two persons with great strength. Please
pull out all power cords before moving.
20. To prevent the harm on patient for accidental moving, please lock the casters during usage.
21. To prevent accidental disconnection, secure all patient leads, infusion lines and ventilator
tubing to the mattress with sufficient excess length to allow for the full range of mattress
height adjustment.
22. To avoid water over flow from water chamber, and keeping the most stable position of infant
incubator, before moving all of accessories should be fixed to the right position and let out all
of the water.
23. Do not switch on for longer time than essential, when the machine not connect to the power
supply, otherwise, power supply failure alarm will be actively and the internal battery current
waste.
24. When using X-Ray tray via incubator hood, the shadow of hood will reflect on the X-ray
negative. That maybe influence the doctor’s diagnose.
25. Do not place any article higher than incubator’s caster under its VHA cabinet which may affect
the stabilization of VHA cabinet.
26. When operating the VHA cabinet, support the incubator with one hand on to prevent it from
unbalance.
27. When replacing the fuse, you must cut off the power supply and replace it according to the
stipulated specification.
28. The device must be fully cleaned and sterilized for the first time for initial use, after nursing for
one baby, after used it for one week or there's dirt in the incubator. Cleaning and sterilizing
methods please refer to Section 5.
29. Please use the neutral deterge/ disinfectant registered by nation. Other disinfectant (like
alcohol) will destroy some parts of the incubator. Please follow the instruction for detergent
usage.
30. The customer uses any parts, accessories, or fittings only specified or sold by our company, or
else, it will reduce the safety of device.
31. Damages will be easily caused if using the incubator after it reached its lifetime. Previous
capability guideline and requirement cannot be reached as well.
32. The device, accessories and the packaging have to be disposed of waste correctly at the end
of the usage. Please follow Local Ordinances or Regulations for disposal.

iv
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/0
ELECTRICAL PRECAUTIONS
1. To ensure grounding reliability, connect the ac power cord only to a properly grounded 3-wire
hospital grade or hospital use outlet. Do not use extension cords. If any doubt exists as to the
grounding connection, do not operate the equipment.
2. An electric shock hazard exists within the Controller and VHA base assembly when the cover is
removed. Servicing should be performed only by qualified personnel with appropriate service
documentation.
3. To prevent equipment damage or accidental power disconnection, Do NOT connect an
Incubator power cord directly to an ac wall socket when the Incubator is mounted on a VHA
stand. Always provide power to the Incubator using the power cord coming directly form the
VHA stand.
4. Make sure the building power source is compatible with the electrical specifications shown on
the rear center column of the VHA stand or on the Incubator.
5. This device adopts mains plug or appliance coupler as isolation form the supply mains when the
Incubator is mounted on a mobile stand, for safety, and please pull off the power cord when
stopping using the device or repair it.
6. Improbably using of the assistant device will cause the decrease of our device’s safety. The
safety of auxiliary devices shall comply with the general requirements for safety according to
IEC601-1, and have acquired the certificate by relative institution.
HUMIDITY PRECAUTIONS
1. The incubator is with the humidity controller, it can increase the Humidity of incubator according
to the clinical demands.
2. Higher relative humidity will, at any given time, decrease an infant’s evaporative water loss and
may cause an increase in infant temperature. Monitor the infant’s temperature as required.
3. Use only distilled water to fill or refill the Reservoir. Tap water may contain organisms that may
flourish in the heated water of the humidifier.
4. Make sure all hood access door gaskets and tubing ports are properly installed. Any open gaps
in the incubator hood will reduce the incubator’s internal relative humidity.
5. Fill the humidity chamber to the bottom of the MAXIUM LIMIT line. DO NOT OVERFILL. Or else
water spillage may result.
6. Following the doctor’s advice when setting the relative humidity.

v
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/0
OXYGEN PRECAUTIONS
1. This incubator is with oxygen control system, please use iatric oxygen when feeding oxygen.
2. Abusing of supplemental oxygen may result in serious aftereffects which include blindness,
brain damage, even death. Therefore, keeping to the main doctor's direction strictly and
monitoring the oxygen supplement condition for the patient in a regularly time.
3. If it is necessary to administer Oxygen in an emergency, notify the attending physician
immediately.
4. When supplementing the oxygen, calibrated oxygen analyzer must be turned on for monitoring
the oxygen concentration.
5. Oxygen feeding may increase the noise level inside the hood.
6. As Oxygen use increases the danger of fire. To ensure the device safety, make all flammable
material far away from incubator, and auxiliary equipment producing sparks should not be
placed near incubator.
7. When the oil, grease, other fat substance and the compressed oxygen meet, it will self-ignite
seriously, therefore, try to avoid the oxygen pressure reducing valve/adjustment valve, valve for
oxygen cylinder, pipe, connector containing these substance.
8. Do not use combustible material like aether, alcohol etc. because once even a little aether,
alcohol, or other combustible material mixed with oxygen in the incubator, it’ll cause fire.
9. When modulating the oxygen flux every time, please leave 30min. at least for the incubator
regaining the new oxygen concentration.
10. There’s a pressurize device filling with potassium hydroxide electrolyte installed inside the
oxygen sensor. If the sensor leaks, please stop using and chuck it. If the leaking electrolyte
touches skin or clothes, please wash with clean water immediately. If the leaking electrolyte
touches eyes, please wash eyes with clean water for 15 min. and keep them open, notify the
doctor immediately.
11. Seasonal check the gas and the oxygen transporting parts to see if they are eroded or broken.
12. Seasonal check the battery of oxygen sensor to if they are leaking or aging. Replace them if it
is necessary.
13. A little flammable material left inside of hood will meet oxygen and cause fire, such as aether or
alcohol.
14. Please refer to the relevant user's manual when the auxiliary oxygen device is used together
with the incubator.

vi
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/0
WEIGHING PRECAUTIONS
1. The electrical scale installed in the bassinet must work after 30 minutes’ warm-up in the
incubator, that is to say the electrical scale can’t work until being put in the incubator which is
set the using temperature and begins working for at least 30min. Or else, the number read on
the scale will surpass the regulated value.
2. Please keep the patient aclinic and in the middle of the bassinet while weighing.
3. The maximum weighing weight of Infant scale is 8kg, please don’t over loading, or else the
scale will be damaged.
4. Lay the bassinet with infant scale gently when loading or unloading, don’t press surface of the
bassinet to avoid damage the weight sensor inside the bassinet.
5. The displayed weighing value is just for reference.
SEASONAL SAFETY CHECK
1. Please clean the plug of power cord at least once a year. Too much dust on plug may cause the
fire.
2. The following safety checks should be performed at least every 12 months by a qualified person
who has adequate training, knowledge, and practical experience to perform these tests. The data
should be recorded in an equipment log.
○
1. Inspect the equipment and accessories for mechanical and functional damage.
○
2. Inspect the safety relevant labels for legibility.
○
3. Inspect the fuse to verify compliance with rated current and breaking characteristics.
○
4. Verify that the device functions properly as described in the instructions for use.
○
5. Test the protection earth resistance according IEC 60601-1:1988 + A1:1991 + A2:1995:
Limit 0.1Ω.
○
6. Test the earth leakage current according IEC 60601-1:1988 + A1:1991 + A2:1995: Limit:
NC 500μA, SFC: 1000μA.
○
7. Test the enclosure leakage current according to IEC 60601-1:1988 + A1:1991 + A2:1995:
Limit: NC 100μA, SFC: 500μA.
○
8. Test the patient leakage current according IEC 60601-1:1988 + A1:1991 + A2:1995: Limit:
for a.c.: 100μA (BF), for d.c.: 10μA (BF).
○
9. Test the patient leakage current under single fault condition with mains voltage on the
applied part according IEC 60601-1:1988 + A1:1991 + A2:1995: Limit: for a.c.:500μA (BF), for d.c.:
50μA (BF).
○
10 . According to the test methods of IEC 60601-1:1988 + A1:1991 + A2:1995, the patient
leakage current (net voltage should be added on the applied part) of the testing device must less
than 5000μA.
○
11 . Test the patient auxiliary leakage current according IEC 60601-1:1988 + A1:1991+
A2:1995: Limit: NC for a.c.: 100μA (BF), for d.c.: 10μA (BF).SFC 500μA (BF), for d.c.: 50μA (BF).

vii
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/0
TABLE OF DEFINITIONS AND SYMBOLS
TECHNICAL DEFINITIONS
SKIN TEMPERATURE SENSOR: A sensing device including the link with the equipment intended
to measure the infant’s skin temperature.
INCUBATOR TEMPERATURE: Air temperature at a point 10cm above and centered over the
mattress surface.
CONTROL TEMPERATURE: The temperature set at the temperature control.
AVERAGE INCUBATOR TEMPERATURE: The average of the maximum and minimum Incubator
temperatures achieved during Temperature Condition.
STEADY TEMPERATURE CONDITION: A condition which is reached when the temperature does
not vary by more than 1℃over a period of 1 hour.
TEMPERATURE ALARM CHECKOUT STATE: The difference between real temperature and
control temperature is within ±0.5℃and such state lasts for over 10 minutes. The equipment must
stay in such state when check up the alarm about temperature.
TEMPERATURE UNIFORMITY: The amount by which the average temperature at each of four
points 10cm above the mattress surface differs from the Average incubator Temperature at steady
Temperature Condition. The four points are the centers of four quadrants formed by lines that
divide the width and length of the mattress surface.
TEMPERATURE VARIABILITY: The variability of the Incubator Temperature that will be observed
over a one hour period after Incubator Temperature Equilibrium has been reached.
TEMPERATURE RISING TIME: The time required for the Incubator Temperature to rise 11℃,
when the Air Control Temperature is at least 12℃above ambient.
STEADY HUMIDITY CONDITION: A condition that the disparity between the indicated humidity
value and control value is less than ±5%RH, and maintains over 2 min.
NOTE, IMPORTANT, CAUTION AND WARNING
NOTE: A note is inserted in text to point out procedures or conditions, which may otherwise be
misinterpreted or overlooked. A note may also be used to clarify apparently contradictory or
confusing situations.
IMPORTANT: Similar to a Note but be used where greater emphasis is required.
CAUTION: A caution is inserted in text to call attention of a procedure which, It not followed
exactly, can lead to damage or destruction of the equipment.
WARNING: A warning is inserted in text to call attention to dangerous or hazardous conditions
inherent to the operation, cleaning, and maintenance of the equipment which may result in
personal Injury or death of the operator or patient.

viii
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/0
SYMBOLS
Attention; Consult
accompanying documents
Protective Earth (Ground)
Class I Equipment Type BF Applied Part
Main power on Main power off
On (only for a part of equipment)
Off (only for a part of equipment)
Fuse Type F 10AH/250V
AC Power 110-120V, 50/60Hz
Weight Limit High pressure, dangerous
RS-232 Connector Mattress Tilt Direction
Socket for skin temperature 1 Socket for skin temperature 2
High temperature warning
Heating Power Indicator
Oxygen supplement indication
mark
Humidifying indication mark
Indicator ON/OFF Switch indicator key
Silence / Reset Key Keypad Key
Set up key/raise device’s height
Set down key/lower device’s height
Air Mode Key
Baby Mode Key
>37 setting key℃
Reset zero key
Calibration key
CE Marking
Water high-level mark Water low-level mark
Serial Number Date Of Manufacture
Assistant net power outlet, MAX:3.15A
CLASS I
F 10AH/250V 110-120V~
50
/
60Hz
110-120V~50/60Hz MAX:3.15A
LOW HIGH
RS-232
SEL
>37℃
ON/OFF
ZERO
CAL.
2
O
SN

ix
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/1
CONTENTS
SECTION PAGE
1. GENERAL INFORMATION…………………………………………………………………………1-1
1.1 Introduction…………………………………………………………………………………………1-1
1.2 Intended use…………………………………………………………………………………………1-1
1.3 Composition of products……………………………………………………………………………1-1
1.4 Description……………………………………………………………………………………………1-1
1.5 Specifications………………………………………………………………………………………1-3
2. INSTALLATION………………………………………………………………………………………2-1
2.1 General………………………………………………………………………………………………2-1
2.2 Unpacking……………………………………………………………………………………………2-1
2.3 Installation……………………………………………………………………………………………2-1
3. FUNCTION DESCRIPTION………………………………………………………………………3-1
3.1 General function description………………………………………………………………………3-1
3.2 Temperature control principle……………………………………………………………………3-1
3.3 Data communication connector…………………………………………………………………3-2
3.4 Alarming and system indication information……………………………………………………3-3
4. OPERATION…………………………………………………………………………………………4-1
4.1 General………………………………………………………………………………………………4-1
4.2 Power supply connection and switch control……………………………………………………4-1
4.3 Controller, indicators………………………………………………………………………………4-2
4.4 Operation checkout procedure……………………………………………………………………4-5
4.5 General operation procedure……………………………………………………………………4-14
5. CLEANING AND MAINTENANCE…………………………………………………………………5-1
5.1 General………………………………………………………………………………………………5-1
5.2 Cleaning………………………………………………………………………………………………5-1
5.3 Sterilization…………………………………………………………………………………………5-10
5.4 Maintenance………………………………………………………………………………………5-10
5.5 Trouble shooting…………………………………………………………………………………5-10
6. OXYGEN CONCENTRATION CONTROL SYSTEM……………………………………………6-1
6.1 General………………………………………………………………………………………………6-1
6.2 Installation of oxygen sensor………………………………………………………………………6-1

x
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/0
6.3 Function description…………………………………………………………………………………6-2
6.4 Connection of oxygen input connector……………………………………………………………6-2
6.5 Operation and calibration procedures……………………………………………………………6-3
6.6 Using of oxygen supply system……………………………………………………………………6-4
6.7 Maintenance…………………………………………………………………………………………6-5
7. HUMIDITY CONTROL SYSTEM……………………………………………………………………7-1
7.1 General………………………………………………………………………………………………7-1
7.2 Function description…………………………………………………………………………………7-1
7.3 Operation and calibration procedures…………………………………………………………7-1
7.4 Using of humidity control system…………………………………………………………………7-2
7.5 Maintenance…………………………………………………………………………………………7-3
8. WEIGHING SYSTEM…………………………………………………………………………………8-1
8.1 General………………………………………………………………………………………………8-1
8.2 Installation……………………………………………………………………………………………8-1
8.3 Function description…………………………………………………………………………………8-2
8.4 Operation and calibration procedures……………………………………………………………8-2
8.5 Operation of weighing system……………………………………………………………………8-2
8.6 Maintenance…………………………………………………………………………………………8-3
NOTE: The product composition maybe different from this manual, but it does not affect
product function. Please understand.
1-1
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/2
SECTION 1
GENERAL INFORMATION
1.1INTRODUCTION
This manual provides instructions for installation, debugging, operation, cleaning and
maintenance of Infant Incubator (incubator). We are not responsible for the malfunction which is
caused due to not following the instruction on our manual.
The operator should read and understand of the content of this manual.
This manual should be put together with the device so as to the client to check at any
moment.
VHA cabinit and weighing system are optional accessories, you can ignore the relative
contents if you haven’t buy them. Neonate jaundice phototherapy is optional assessory, if you buy
it with the incubator, please refer to the operation manual of Neonate jaundice phototherapy to
see the relative operation.
1.2 INTENDED USE
The Infant Incubator is intended to provide a controlled thermal environment and isolation
from ambient air for premature and neonatal infants. The infant incubator is not intended for the
transport of infants.
1.3 COMPOSITION OF PRODUCTS
The infant incubator consists of four components: the double wall hood, the base, the mobile
cabinet, and the controller. The sponge based mattress positions centrally within the confines of
the hood.
1.4 DESCRIPTION
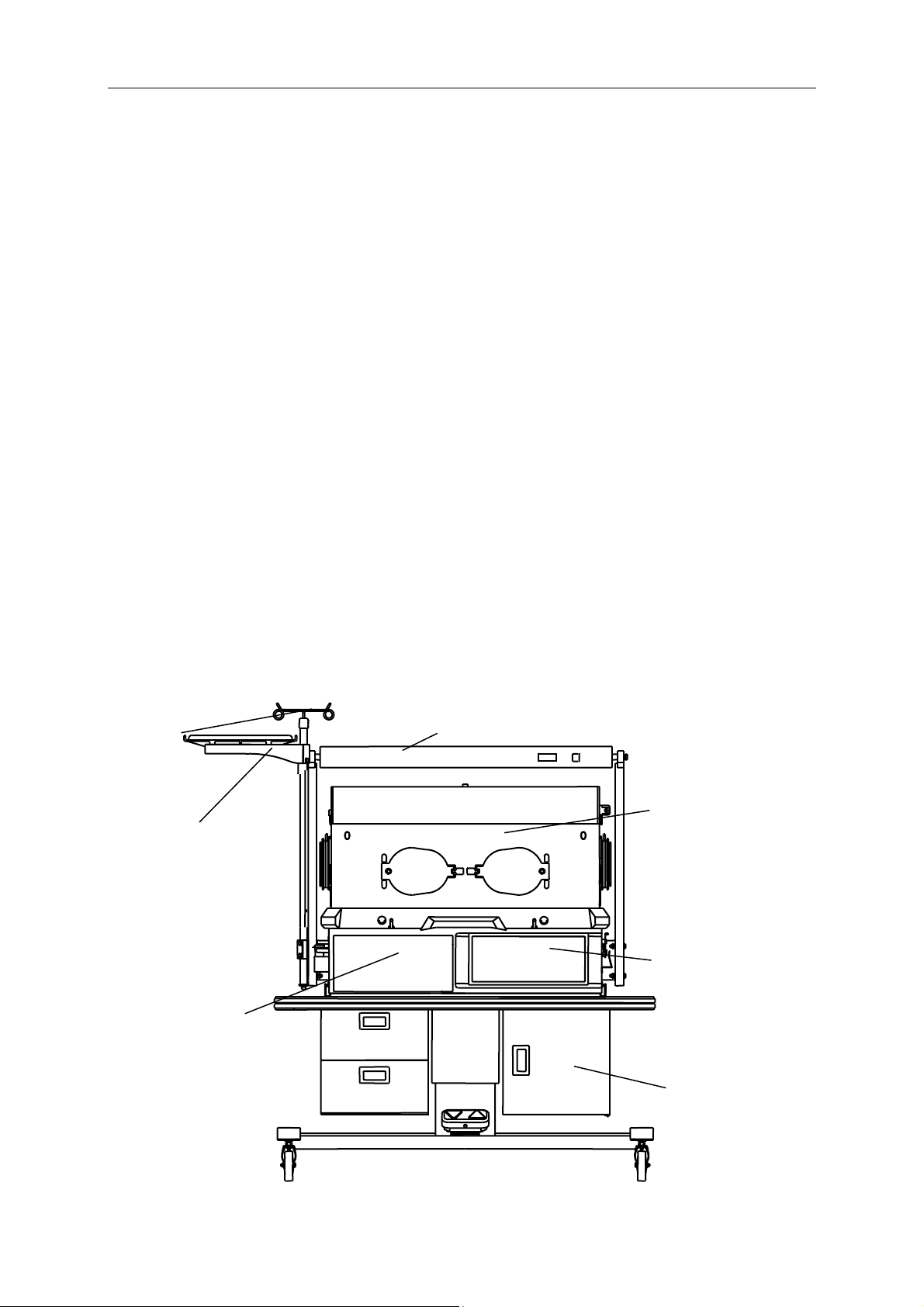
The following diagram shows the main parts of the Infant Incubator.
Neonate Bilirubin Phototherapy Equipment
I.V. Pole
Baby compartment
Shelf
Controller
Base
Mobile Cabinet
1-2
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/0
DESCRIPTION OF PART EXPLANATION
I.V. pole A kind of bearing part, which is used for hanging the infusion
bottle.
Max. Load: 2Kg
Controller
The core part with two kinds of temperature control modes: air
mode, baby mode, moreover, it also have the function of weighing,
humidity control and oxygen concentration controling system
which are used for autocontrol the heat output and the huminidy
and oxygen concentration in the incubator. The detail operation to
temperature please refer to the section 4, to oxygen concentration
please refer to the section 6, to huminidy please refer to the
section 7, to weighing please refers to the section 8.
Shelf A kind of bearing part, which is used for putting some small
objects.
Max. Load: 3.5Kg
Baby compartment
It is used for place the infant inside, including the Acrylic hood,
bassinet, and so on. The bassinet can be tilted at the request of
clinical needs. The baby scale is optional part, and the Max. Load
of bassinet is 10 Kg.
Size of mattress: 630mm × 355mm
Base An important part of Infant Incubator, and it is mainly composed of
the Aluminium tank, humidity chamber, air filter, and so on.
Mobile Cabinet
A part which can support the main body of Infant Incubator. We
have two kinds of cabinet: fixed type and vertical height
adjustment type. Usually we equip the former one, and the second
one is optional part.
Neonate bilirubin
phototherapy
equipment
It is intended for treating the bilirubin of patient.
This part is mounted on top of Infant Incubator, and its light source
has two kinds: fluorescent lamp and LED lamp, the user can
choose this.
for the Neonate Bilirubin Phototherapy Equipment with fluorescent
lamp, please refer to the user’s manual XHZ-90, while for the
Neonate Bilirubin Phototherapy Equipment with LED lamp, please
refer to the user’s manual XHZ-90L.
NOTE: Size of Infant Incubator:
W650 × H1560 × D1440mm
(for Infant Incubator with fixed cabinet);
W650 × H1500 × D1440mm ~ W650 × H1640 × D1440mm
(for Infant Incubator with vertical height adjustment cabinet).
Distance from the bassinet to the floor:
980mm (for Infant Incubator with fixed cabinet);
950mm ~ 1090mm (for Infant Incubator with vertical height adjustment cabinet).
Weight of Infant Incubator with fixed cabinet: 116Kg
Weight of Infant Incubator with vertical height adjustment cabinet: 126Kg
1-3
I4-1
OPERATOR’SMANUALFORINFANTINCUBATOR EDITION/REVISION B/4
1.5 SPECIFICATIONS
Specifications for the Infant Incubator are provided in table 1.1.
TABLE 1.1 SPECIFICATIONS
This equipment belongs to Class I, Type BF applied part, normal device (IP20) continuously
operated.
Power Requirements…………………………………………………AC220-230V, 50Hz, 1000VA
Maximum Heater Power Output……………………………………………………………377W/240V
Auxiliary Mains Power Output……………………AC220V-230V/50Hz, MAX. CURRENT 1.6A
Heater power display……………………………………0 to 100%, adjustable in 10% increments
Temperature control modes…………………………………………………………………Air mode
Baby mode
Air Temperature Control range………………….……………………………………………25℃~37℃
override mode: 37℃~39℃
Baby Temperature Control range ……………………………..…………………………34℃~37℃
override mode: 37℃~38℃
Temperature sensor display range……………………………………………………5℃~65℃
Temperature rise Time*(environment temperature is +22 ) …………………℃.…….…… ≤30min
Temperature Variation*…………………………………………………………………………≤0.5℃
Temperature Uniformity*(level mattress) ………………………………………………………≤0.8℃
Temperature Uniformity *(Tilt mattress) ……………………………………………………≤1.0℃
Accuracy of skin temperature sensor………………………………………………………±0.3℃
Deviation between the indicated air temperature and the real temperature………………≤0.8℃
ALARM(See table 3.1)………………………………………………………Power failure alarm
Fan motor alarm
Sensor failure alarm
Hig deviation alarm
Low deviation alarm
Over temperature alarm
The sensor box position alarm
Low water alarm
Tank position alarm
System alarm
Table of contents
Other NINGBO DAVID Accessories manuals