Pag. 1/67
1. GENERAL INFORMATIONS ..................................................................................... 3
1.1 THANKS FOR CHOOSING THE MEDICAL DEVICE .................................................................. 3
1.2 CONTACT DETAILS FOR ASSISTANCE ................................................................................... 3
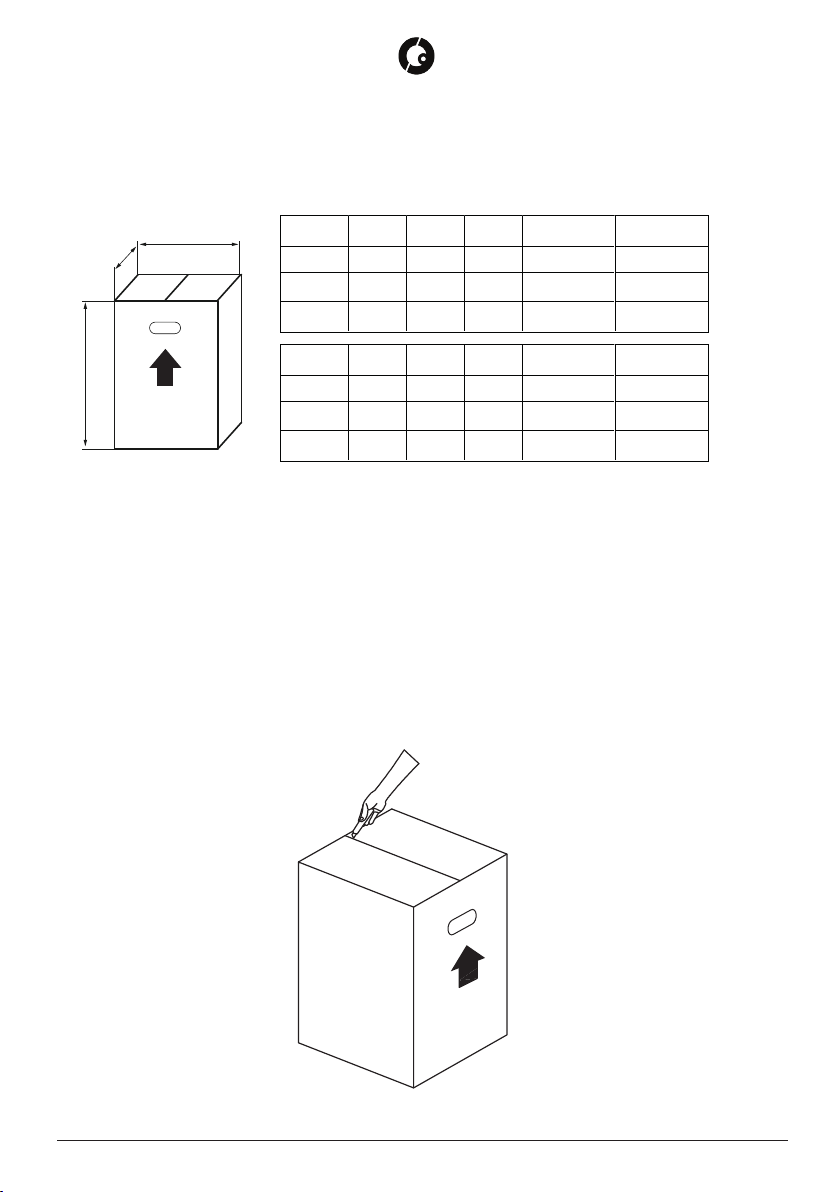
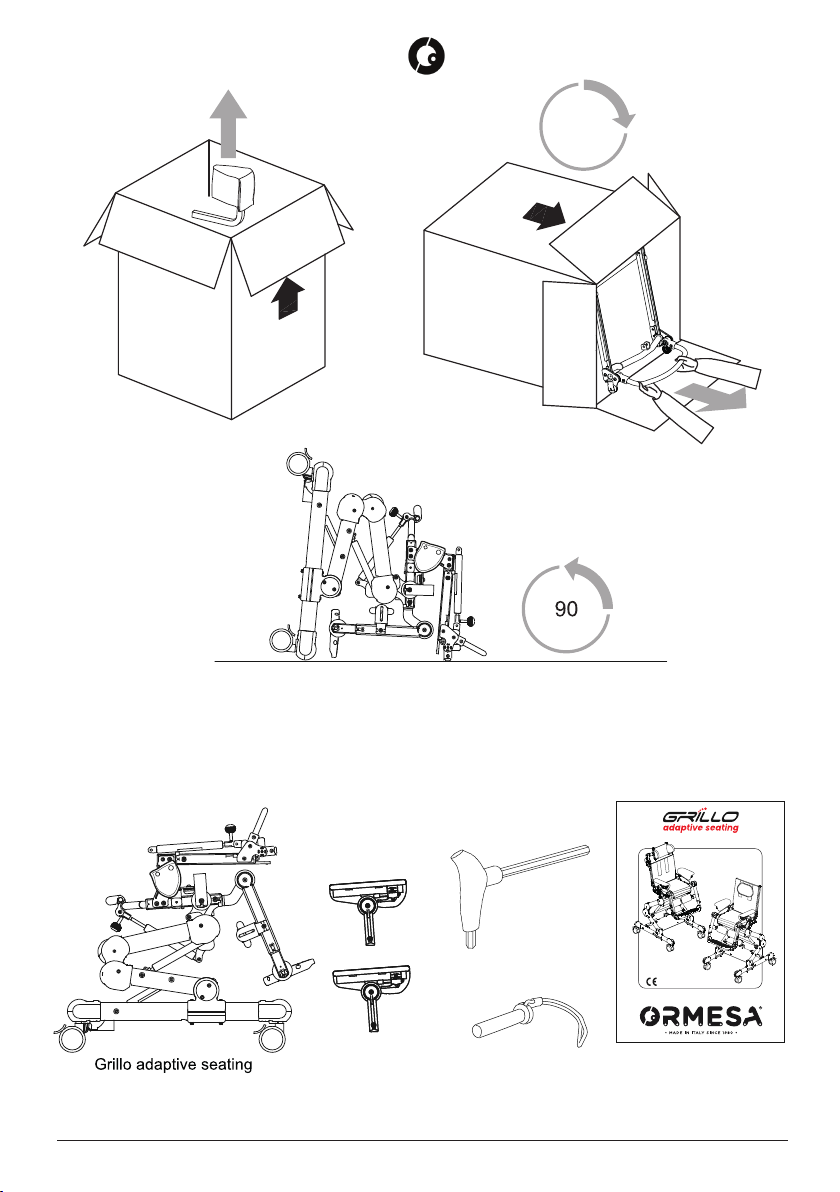
1.3 PACKAGING INFORMATION. UNPACKING INSTRUCTIONS AND SUPPLY COMPOSITION .... 4
1.4 MECHANICAL AND DIMENSIONAL CHARACTERISTICS ........................................................ 6
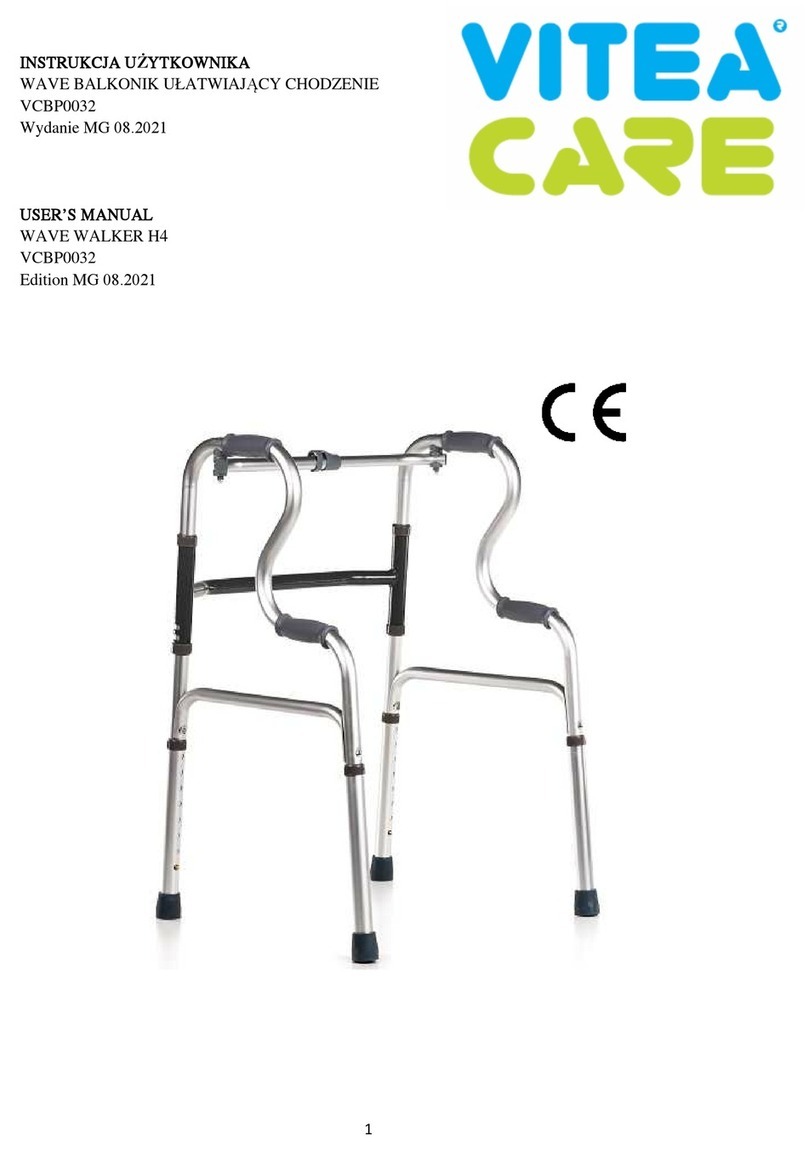
1.5 PRODUCT PARTS LEGEND .................................................................................................... 7
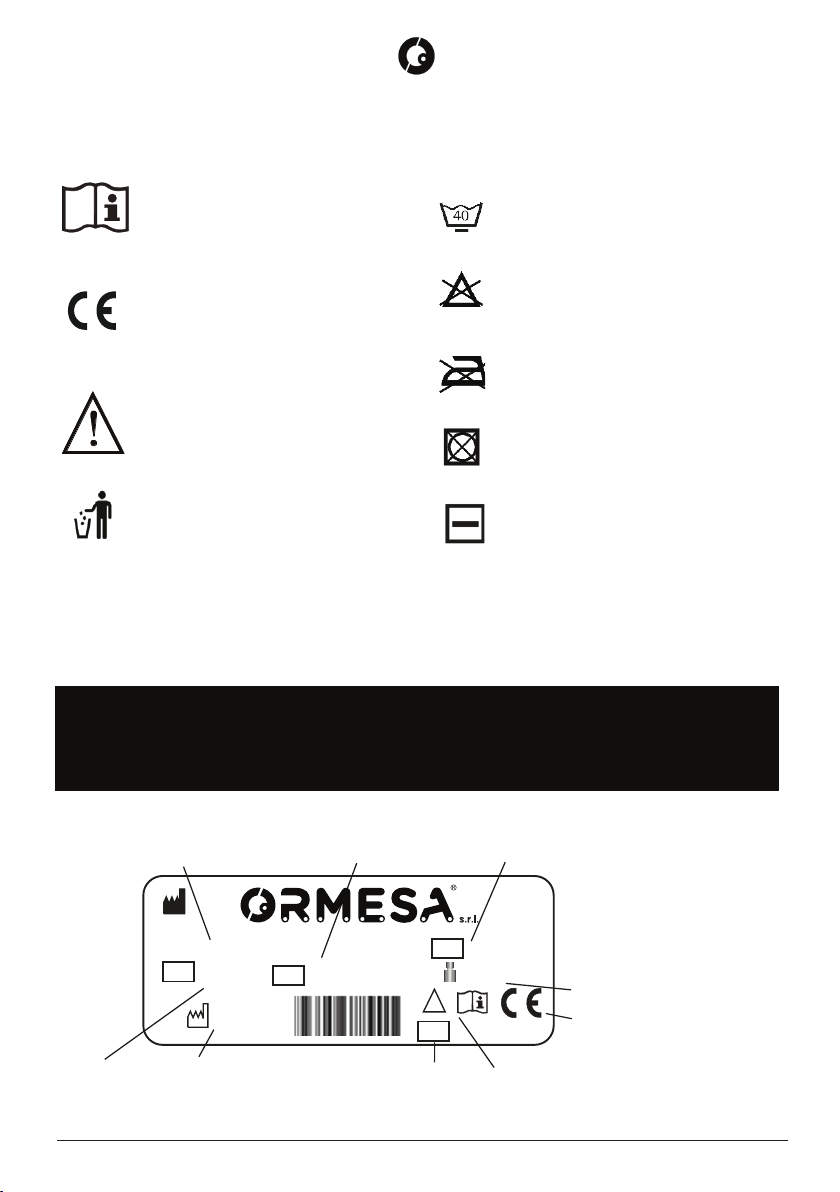
1.6 SYMBOLOGY USED IN THE MANUAL ................................................................................... 8
1.7 IDENTIFICATION PLATE ....................................................................................................... 8
2. LEGAL AND REGULATORY REFERENCES .................................................................. 9
2.1 LEGAL REFERENCES ............................................................................................................. 9
2.2 REGULATORY REFERENCES ................................................................................................. 9
2.3 WARRANTY CONDITIONS .................................................................................................. 10
3. SAFETY WARNINGS .............................................................................................. 10
3.1 MEDICAL DEVICE RISK CLASS ACCORDING TO ANNEX VIII OF REGULATION (EU)
745/2017 ........................................................................................................................... 10
3.2 GENERAL WARNINGS ........................................................................................................ 10
3.3 SPECIFIC WARNINGS ......................................................................................................... 11
3.4 REASONABLY FORESEEABLE MISUSE ................................................................................ 11
3.5 CONTRAINDICATIONS AND SIDE EFFECTS ......................................................................... 12
3.6 OPERATING ENVIRONMENTAL CONDITIONS .................................................................... 12
3.7 CONDITIONS OF TRANSPORT AND PACKAGING ................................................................ 12
3.8 PRE-INSTALLATION/INSTALLATION AND COMMISSIONING ............................................. 13
4. DEVICE DESCRIPTION ........................................................................................... 13
4.1 INTENDED USE OF THE MEDICAL DEVICE .......................................................................... 13
4.2 MAINS COMPONENTS/AVAILABLE VERSIONS ................................................................... 13
4.3 DESCRIPTION OF THE MEDICAL DEVICE ............................................................................ 13
5. OPERATING INSTRUCTIONS ................................................................................. 14
5.1 FIRST USE ........................................................................................................................... 14
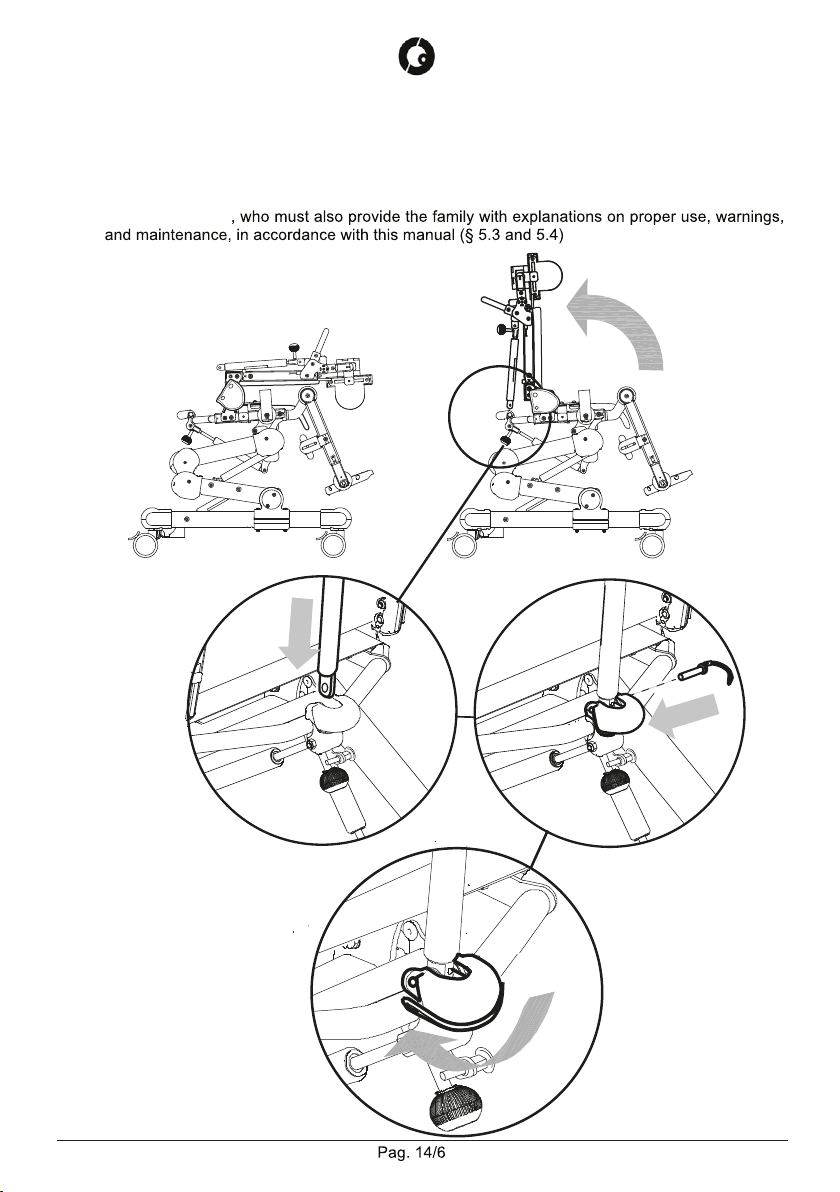
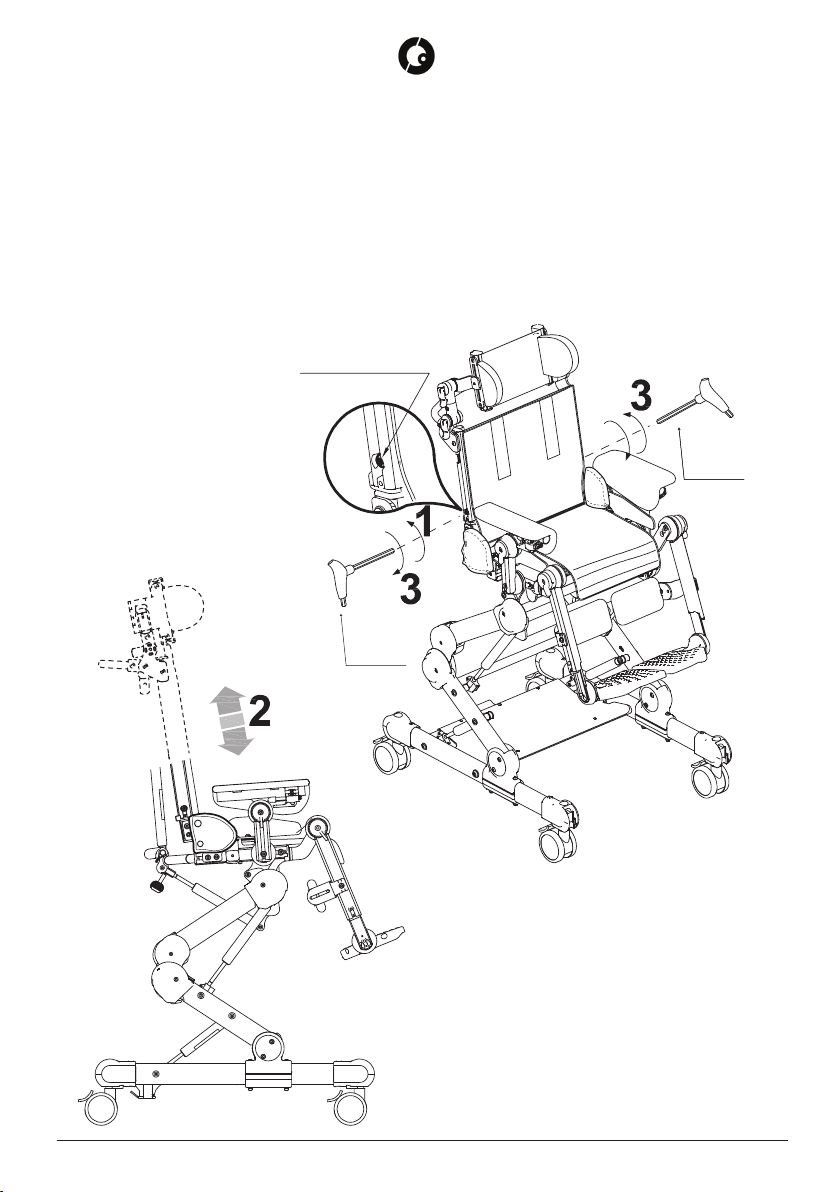
5.2 ASSEMBLY ......................................................................................................................... 14
5.3 ADJUSTMENT AND CONFIGURATION OF THE MEDICAL DEVICE by the health
professional ....................................................................................................................... 16
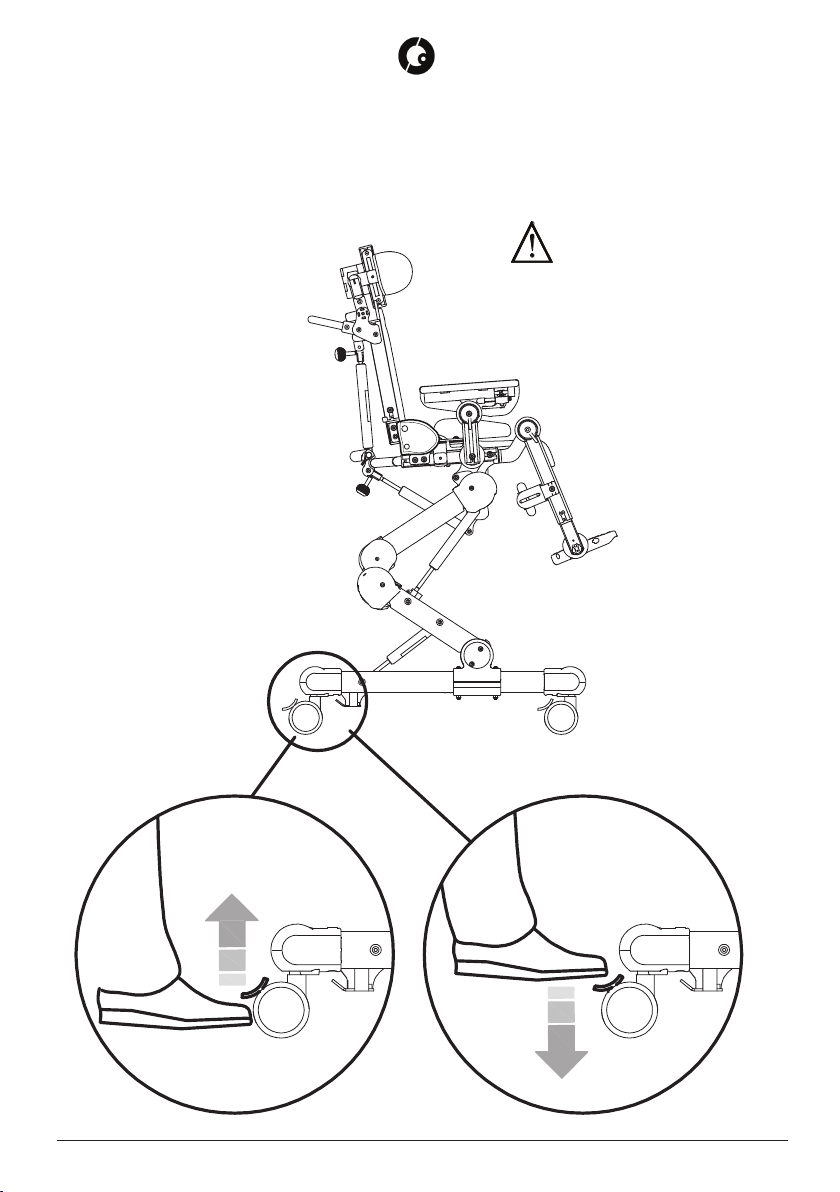
BRAKING ............................................................................................................................ 16
BACKREST HEIGHT ADJUSTMENT ...................................................................................... 17
BACKREST INCLINATION ADJUSTMENT ............................................................................. 18
863 HEADREST ADJUSTMENT ............................................................................................ 19
SEAT TILT ADJUSTMENT (TILT IN SPACE) ........................................................................... 21
SEAT DEPTH ADJUSTMENT ................................................................................................ 23
PELVIC SIDE SUPPORTS ADJUSTMENT .............................................................................. 24
ARMREST ADJUSTMENT .................................................................................................... 26
CALF RESTS ADJUSTMENT ................................................................................................. 28
LEGREST ADJUSTMENT ..................................................................................................... 30
FOOTRESTS ADJUSTMENT ................................................................................................. 32
5.4 ADJUSTMENT AND CONFIGURATION OF THE ADDITIONAL COMPONENTS BY THE
HEALTH PROFESSIONAL .................................................................................................... 33