CAUTION
*Do not inflate the cuff on the samb limb which other monitoring ME equipment is
applied around simultaneously, because this could cause temporary loss of function of
those simultaneously-used monitoring ME equipment.
*On the rare occasion of a fault causing the cuff to remain fully inflated during
measurement, open the cuff immediately. Prolonged high pressure (cuff pressure >
300mmHg or constant pressure > 15mmHg for more than 3 minutes) applied to the
wrist may lead to an ecchymosis.
*Please check that operation of the device does not result in prolonged impairment of
patient blood circulation.
* When measuring, please avoid compression or restriction of the connection tubing.
* The device cannot be used with HF surgical equipment at the same time.
* The ACCOMPANYING DOCUMENT shall disclose that the SPHYGMOMANOMETER
was clinically investigated according to the requirements of ISO 81060-2:2013.
* To verify the calibration of the AUTOMATED SPHYGMOMANOMETER, please
contact the manufacturer.
* This device is contraindicated for any female who may be suspected of, or is
pregnant. Besides providing inaccurate readings, the effects of this device on the fetus
are unknown.
* Too frequent and consecutive measurements could cause disturbances in blood
circulation and injuries.
* This unit is not suitable for continuous monitoring during medical emergencies or
operations.Otherwise, the patient’s wrist and fingers will become anaesthetic, swollen
and even purple due to a lack of blood.
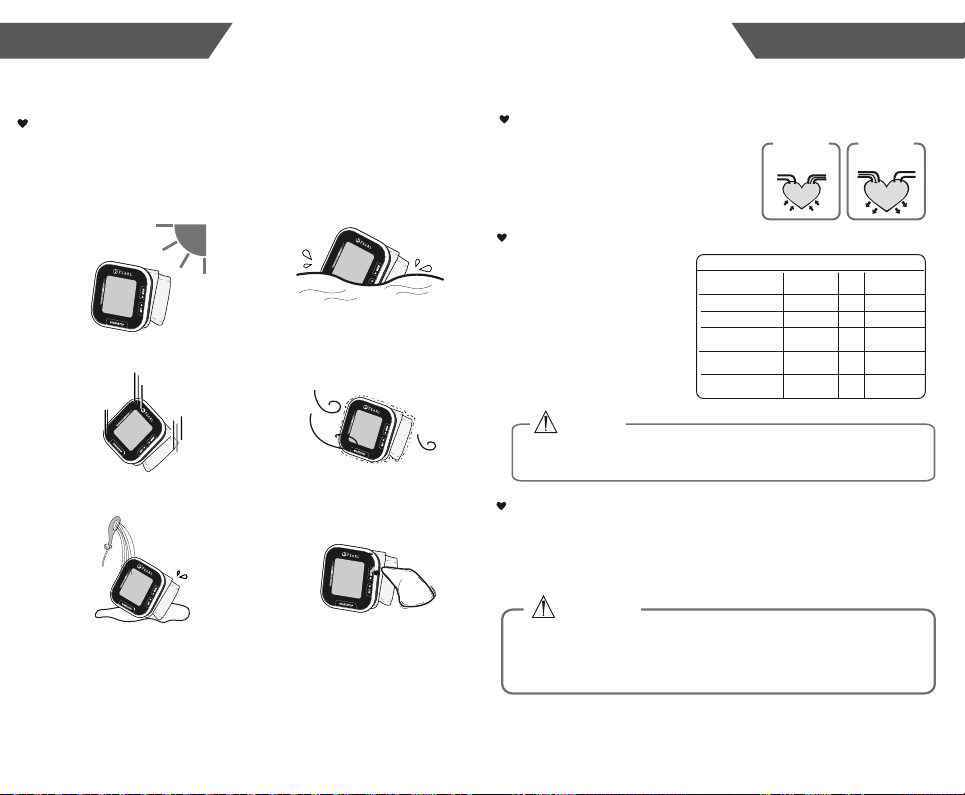
* When not in use, store the device in a dry room and protect it against extreme
moisture, heat, lint, dust and direct sunlight. Never place any heavy objects on the
storage case.
* This device may be used only for the purpose described in this booklet. The
manufacturer cannot be held liable for damage caused by incorrect application.
*This device comprises sensitive components and must be treated with caution.
Observe the storage and operating conditions described in this booklet.
* The maximum temperature that the applied part can be achieved is 42.5℃while the
environmental temperature is 40℃.
* The equipment is not AP/APG equipment and not suitable for use in the presence of a
flammable anesthetic mixture with air of with oxygen or nitrous oxide.
* Warning: No servicing/maintenance while the ME equipment is in use.
* The patient is an intended operator.
* The patient can measure data and change battery under normal circumstances and
maintain the device and its accessories according to the user manual.
* To avoid measurement errors, please avoid the condition of strong electromagnetic
field radiated interference signal or electrical fast transient/burst signal.
* The blood pressure monitor and the cuff are suitable for use within the patient
environment. If you are allergic to polyester, nylon or plastic, please don't use this
device.
* During use, the patient will be in contact with the cuff. The materials of the cuff have
been tested and found to comply with requirements of ISO 10993-5:2009 and ISO
10993-10:2010. It will not cause any potential sensization or irritation reaction.
* If you experience discomfort during a measurement, such as pain in the wrist or other
complaints, press the START/STOP button to release the air immediately from the cuff.
Loosen the cuff and remove it from your wrist.
* If the cuff pressure reaches 40 kPa (300 mmHg), the unit will automatically deflate.
Should the cuff not deflate when pressures reaches 40 kPa (300 mmHg), detach the
cuff from the wrist and press the START/STOP button to stop inflation.
* Before use, make sure the device functions safely and is in proper working condition.
Check the device, do not use the device if it is damaged in any way. The continuous
use of a damaged unit may cause injury, improper results, or serious danger.
* Do not wash the cuff in a washing machine or dishwasher!
* The service life of the cuff may vary by the frequency of washing, skin condition, and
storage state. The typical service life is 10000 times.
* It is recommended that the performance should be checked every 2 years and after
maintenance and repair, by retesting at least the requirements in limits of the error of
the cuff pressure indication and air leakage (testing at least at 50mmHg and
200mmHg).
* Please dispose of ACCESSORIES, detachable parts, and the ME EQUIPMENT
according to the local guidelines.
* Manufacturer will make available on request circuit diagrams, component part lists,
descriptions, calibration instructions,etc., to assist to service personnel in parts repair.
* The operator shall not touch output of batteries and the patient simultaneously.
* Cleaning :Dust environment may affect the performance of the unit. Please use the
soft cloth to clean the whole unit before and after use. Don’t use any abrasive or volatile
cleaners.
* The device doesn’t need to be calibrated within two years of reliable service.
* If you have any problems with this device, such as setting up, maintaining or using,
please contact the SERVICE PERSONNEL of Pearl. Don’t open or repair the device by
yourself in the event of malfunctions. The device must only be serviced, repaired and
opened by individuals at authorized sales/service centers.
* Please report to Pearl if any unexpected operation or events occur.
* Keep the unit out of reach of infants, young children or pets to avoid inhalation or
swallowing of small parts. It is dangerous or even fatal.
* Be careful to strangulation due to cables and hoses, particularly due to excessive
length.
* At least 30 min required for ME equipment to warm from the minimum storage
temperature between uses until it is ready for intended use. At least 30 min required for
ME equipment to cool from the maximum storage temperature between uses until it is
ready for intended use.
* This equipment needs to be installed and put into service in accordance with the
information provided in the ACCOMPANYING DOCUMENTS;
* Wireless communications equipment such as wireless home network devices, mobile
phones, cordless telephones and their base stations, and walkie-talkies can affect this
equipment and should be kept at least a distance d away from the equipment. The
distance d is caculated by the MANUFACTURER from the 800 MHz to 2.5 GHz column
of Table 6 of IEC 60601-1-2:2007, as appropriate.
* Please use ACCESSORIES and detachable partes specified/ authorised by
MANUFACTURE. Otherwise, it may cause damage to the unit or danger to the
user/patients.
* There is no luer lock connectors are used in the construction of tubing, there is a
possibility that they might be inadvertently connected to intravascular fluid systems,
allowing air to be pumped into a blood vessel.
* Please use the device under the environment which was provided in the user manual.
Otherwise, the performance and lifetime of the device will be impacted and reduced.
CAUTION
4
INTRODUCTION INTRODUCTION
5