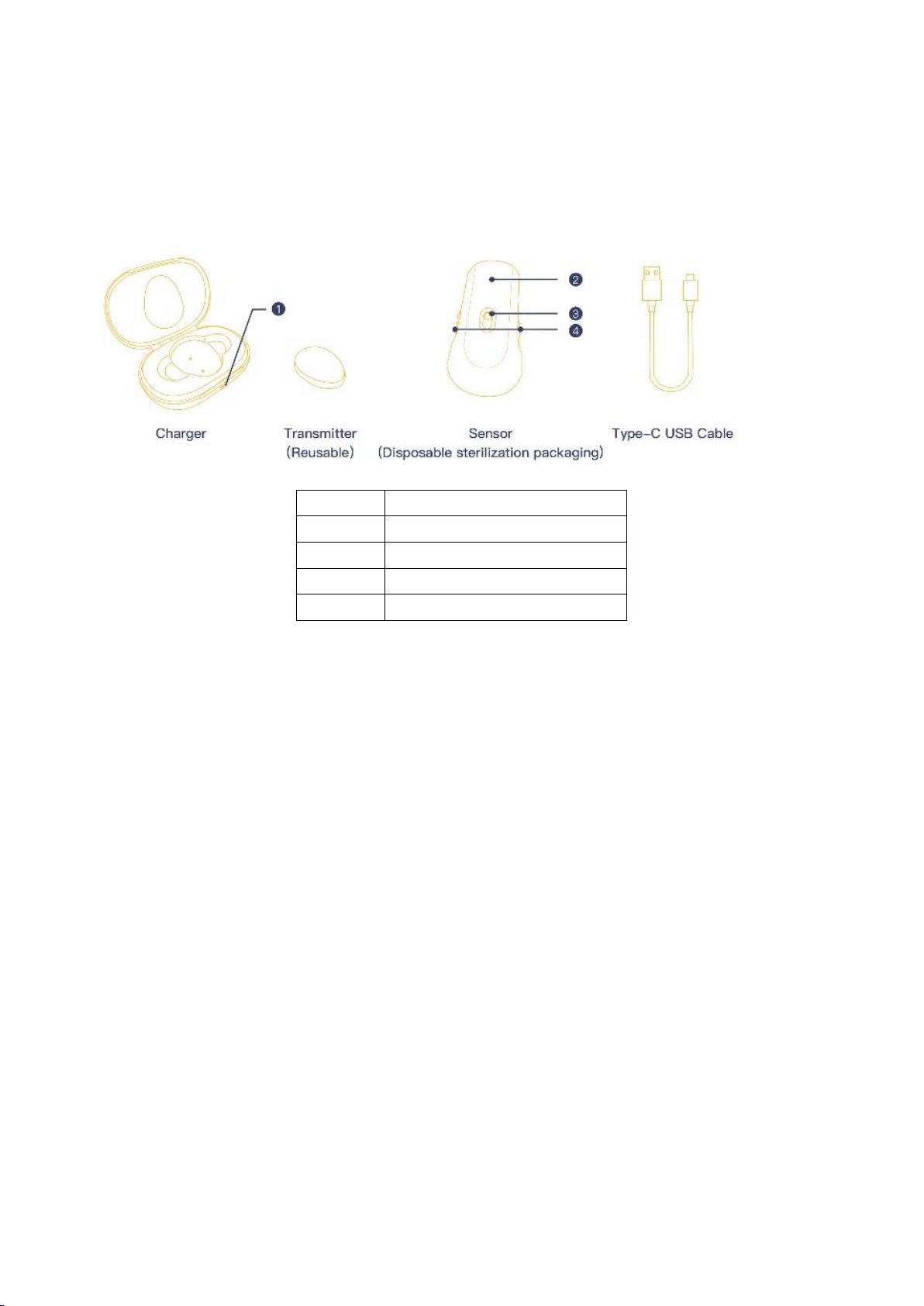
1
1. Introduction
1.1 Foreword
Dear Users,
Please make sure that you carefully read the User Manual included with your CT3 series real time
continuous glucose monitoring (rtCGM) system before use to gain a full understanding of the instructions for use
and all indications, contraindications, warnings, precautions and cautions. Always keep the User Manual for future
reference. If you have any questions or difficulties regarding the use of your CT3 series, please do not hesitate to
contact us. You are encouraged to report any events of concern to us.
An "event of concern" refers to an event that directly or indirectly had, would have, could have, or may have
any of the following consequences:
•harm to the user or any other person;
•a serious public health hazard.
You will find our contact information in Imprint at the beginning of the User Manual.
1.2 What the User Manual is for?
The instructions described in the User Manual is for the use of POCTech’s CT3 series(contains CT3,CT3A,CT3C)
rtCGM. The contents of the User Manual are subject to change without notice.
1.3 Limits on POCTech’s liability obligations
POCTech shall not be liable for any personal injury or damage caused by failure to use the CT3 series rtCGM
System and its components according to the instructions for use and all indications, contraindications, warnings,
precautions, and cautions, including but not limited to:
improper use (e.g., use with protective covers removed, use without indications etc.)
improper maintenance (e.g., intentional damage to cables or electrodes, unauthorized repairs or
modifications etc.)
1.4 Label symbols
A WARNING provides important information about a potentially hazardous situation that, if not avoided, can
have serious consequences.
A CAUTION provides important information about a potentially hazardous situation which, if not avoided, may
result in minor or moderate injury to the user or damage to the medical device or other property.
A NOTE contains additional information to avoid malfunctions during operation.
1.5 eIFU
You can get the latest version of User Manual at www.poctechcorp.com.
1.6 Software basic information
1.6.1Software name:AnytimeWell,AnytimeFollow
1.6.2 Software operating environment