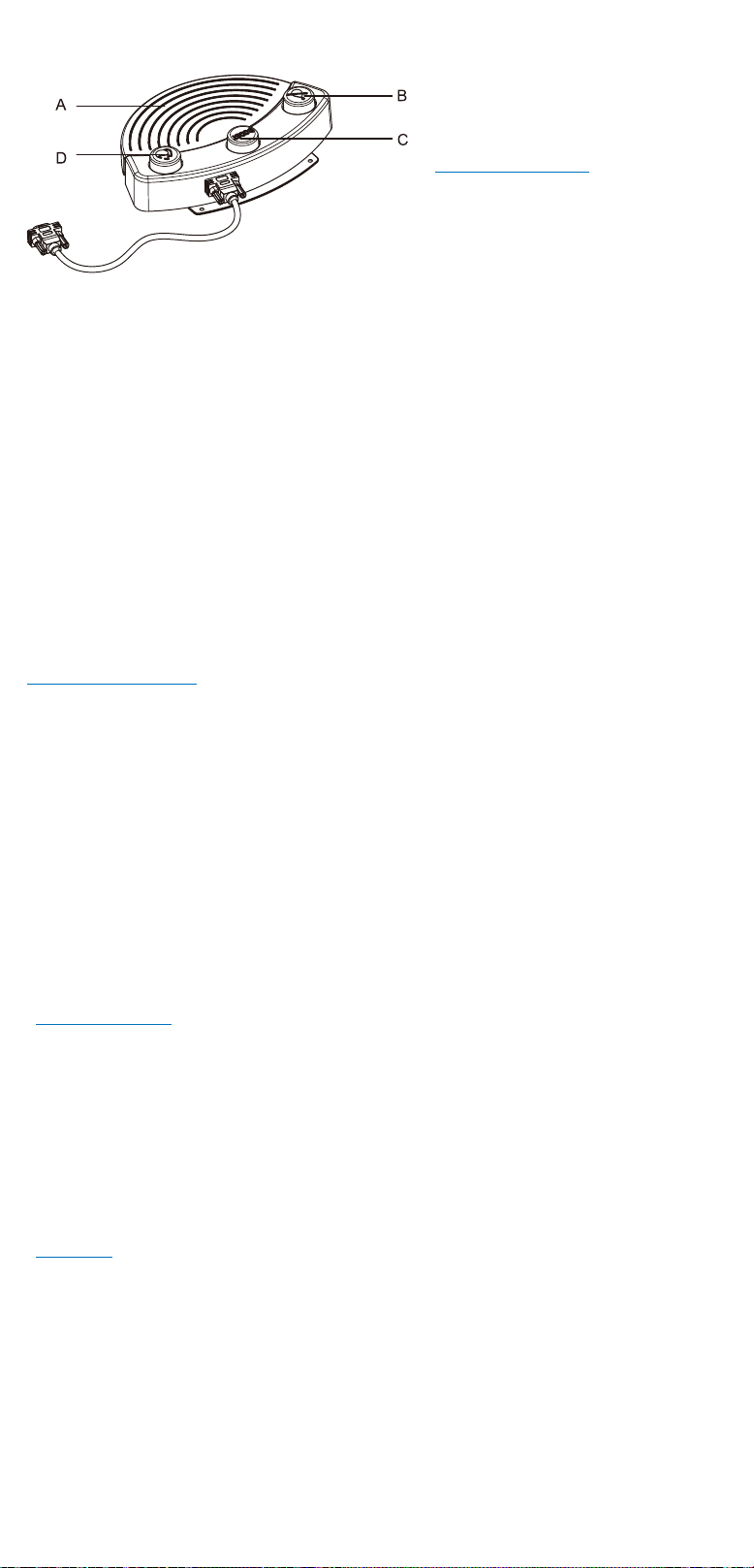7
4.2 Notes
Note1 If the tip is surely tightened and there is water mist spray, the tip is considered to be damaged with the following phenomena:
1) The vibration intensity of the tip and the degree of water atomization are significantly weakened.
2) The tip makes a harsh click sound during operation.
5 Cleaning, disinfection, and sterilization
5.1 Initial processing
5.1.1 Processing principles
It is only possible to carry out effective sterilization after the completion of effective cleaning and disinfection. Please ensure that,
as part of your responsibility for the sterility of products during use, only sufficiently validated equipment and product-specific
procedures are used for cleaning/disinfection and sterilization, and that the validated parameters are adhered to during every cycle.
Please also observe the applicable legal requirements in your country as well as the hygiene regulations of the hospital or clinic,
especially with regard to the additional requirements for the inactivation of prions.
5.1.2 Post-operative treatment
The post-operative treatment must be carried out immediately, no later than 30 minutes after the completion of the operation. The
steps are as follows:
Let the device works for 20-30 seconds at maximum water volume to separately
flush the Ultrasonic handpiece, tip, Air polishing handpiece, and nozzle;
Remove the handpieces from the device and rinse away the dirt on the surface of handpieces and their accessories (tip, nozzle and
torque wrench) with pure water (or distilled water/deionized water);
Dry the handpieces and its accessories with a clean, soft cloth and place it in a clean tray.
Precautions:
The water used here must be pure water, distilled water or deionized water.
5.2 Cleaning
The cleaning of handpiece and its accessories should be performed no later than 24 hours after the operation.
The cleaning can be divided into automated cleaning and manual cleaning. Automated cleaning is preferred if conditions permit.
5.2.1Automated cleaning
△ The cleaner is proved to be valid by FDA, CE certification or in accordance with EN ISO 15883.
△ There should be a flushing connector connected to the inner cavity of the product. The cleaning procedure is suitable for the
handle, and the flushing period is sufficient. But ultrasonic cleaning is not allowed for Ultrasonic handpiece.
△ It is recommended to use a washer-disinfector in accordance with EN ISO 15883. For the specific procedure, please refer to the
automated disinfection section in the section Disinfection.
Precautions:
1) The cleaning agent does not have to be pure water. It can be distilled water, deionized water or multi-enzyme. But please ensure
that the selected cleaning agent is compatible with the handpiece.
2) The water temperature should not exceed 45, otherwise the protein will solidify and it is difficult to remove.
5.2.2 Manual cleaning
△ Soak the handpieces and its accessories in a cleaning agent (such as multi-enzyme). The soaking time and concentration should
at least reach the time and concentration specified by the detergent manufacturer;
△ Carefully clean the surface of the handpieces and its accessories with a disposable
soft cloth or soft brush to remove any visible dirt on the surface;
△ Rinse the handpieces and its accessories under clean running water (desalted water, distilled water or deionized water) for at least
5 times with duration of no less than 60 seconds for each time.
△ Check whether the cleaned parts are clean or damaged. If the cleaning is not complete, repeat the previous cleaning procedures.
△ The intrinsic suitability of the handpieces and its accessories for effective cleaning using the above procedure was verified by a
validated facility.
Precautions:
1) The cleaning agent used here must be compatible with the handpieces and only freshly prepared solutions can be used.
2) The water temperature should not exceed 45, otherwise the protein will solidify and it is difficult to remove.
5.3 Disinfection
Disinfection must be performed no later than 2 hours after the cleaning phase. Auto- mated disinfection is preferred if conditions
permit.
5.3.1Automated disinfection
If possible, the disinfection cycle should be in accordance with EN ISO 15883. Plea- se ensure that the following standards are met
when selecting a sterilizer system:
△ The sterilizer is FDA approved, CE certified or in accordance with EN ISO 15883.
△ Use high temperature disinfection function. The temperature does not exceed 134 ℃ . The temperature cannot exceed 20 minutes.
△ The sterilizer has a flush connecting piece that is connected to the interior of the handpiece.
△ The cleaning procedure is suitable for the handle and the flushing cycle is sufficient (5-10 minutes).
△ Only distilled or deionized water with a small amount of microorganisms (<10 cfu/ml) can be used for all rinsing steps. (For
example, pure water that is in accordance with the European Pharmacopoeia or the United States Pharmacopoeia).
△ The air used for drying must be filtered by HEPA.
△ Regularly repair and inspect the disinfector.
Cleaning and disinfecting steps by using Washer-disinfector
Carefully place the handpieces and its accessories in the disinfection basket. Fastening of the handpieces and its accessories if only
permissible of they are freely moveable in the fixture. The handpieces and its accessories are not permitted to make contact with one
another.
Use a suitable rinsing adaptor, and attach the handpieces to the rinsing connections of the washer-disinfector so that the surface
and internal water lines can be flushed du- ring cleaning process.
Start the program. After the program is finished, remove the handpieces and its accessories from the washer-disinfector, inspect
(refer to section Inspection and Maintenance) and packaging (refer to chapter Packaging). Dry the handpieces and its accessories
repeatedly if necessary (refer to section Drying).
The intrinsic suitability of the handpieces and its accessories for effective cleaning and disinfection using the above automated
cleaning and disinfection procedures was verified by a certified facility.
Precautions:
1) Before use, you must carefully read the operating instructions provided by the equipment manufacturer to familiarize yourself with
the disinfection process and pre- cautions.
2) With this equipment, cleaning, disinfection and drying will be carried out together.
3) Cleaning: (a) The water temperature should not exceed 45, otherwise the protein will solidify and it is difficult to remove. (b) The
solution used can be pure water, distilled water, deionized water or multi-enzyme solution, etc., and only freshly prepared solutions
can be used. (c) The cleaning agent must be compatible with the handpiece. Please follow the concentration and contact time
provided by manufacturer.