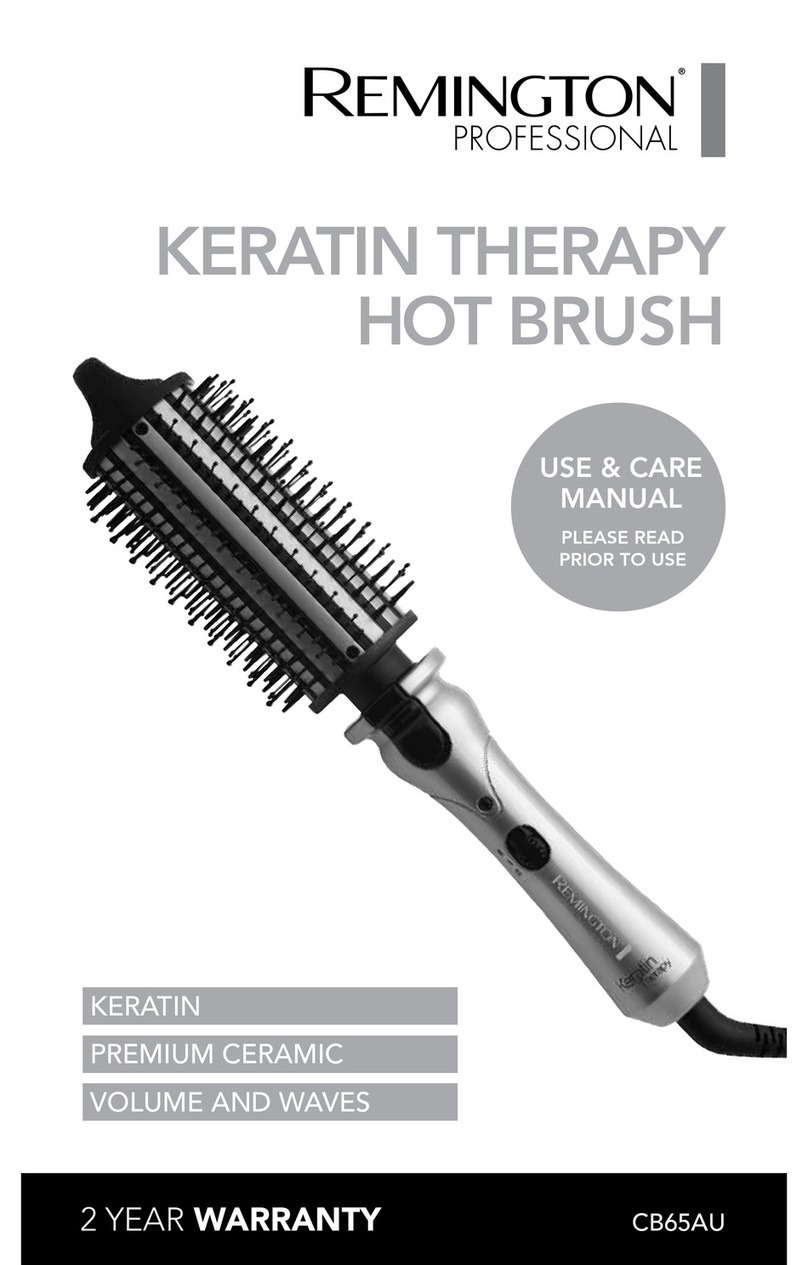
RITM OKB ZAO RITMSCENAR Super Pro v.2 User manual

RITM OKB ZAO
TRANSDERMAL ELECTRONEUROSTIMULATOR
RITMSCENAR Super Pro v.2
OPERATING MANUAL
MANUFACTURER
RITM OKB ZAO
99, Petrovskaya str., Taganrog, Russia, 347900
Tel/Fax: +7 (8634) 62-31-79
www.scenar.com.ru e-mail: medsc@scenar.com.ru
AUTHORIZED REPRESENTATIVE
SCENAR Center –Bulgaria ltd.
9, V. Aprilov blvd. Plovdiv, 4002, Bulgaria

Operating Manual Version 7.3-03 of 28.07.20
2
IMPORTANT INFORMATION!
PLEASE READ THIS PAGE CAREFULLY
WARNING! This device should NOT be used on an individual who has a heart pace-
maker or other electrically powered implant fitted.
WARNING! Application of electrodes near the thorax may increase the risk of cardiac
fibrillation.
WARNING! Simultaneous connection of a patient to a h.f. surgical equipment may result
in burns at the site of the stimulator electrodes and possible damage to the stimulator.
WARNING! Operation in close proximity (e.g. 1 m) to a shortwave or microwave therapy
equipment and mobile communicators may produce instability in the stimulator output.
WARNING! Aged people, children, and people with disabilities may not use the stimulator.
WARNING! The device needs special precautions regarding EMC and needs to be in-
stalled and put into service according to the EMC information provided in Annex 1.
WARNING! As the current densities for electrodes exceeds 2 mA r.m.s./cm2, the device
requires the special attention of the user.
WARNING! The device should not be used adjacent to or stacked with other equipment.
This appliance is marked according to the European directive 2002/96/EC on Waste
Electrical and Electronic Equipment (WEEE). By ensuring this product is disposed of cor-
rectly, you will help prevent potential negative consequences for the environment and hu-
man health, which could otherwise be caused byinappropriatewaste handling of thisproduct.
The symbol on the documents accompanying the product, indicates that this
appliance may not be treated as household waste. Instead it shall be handed over to the
applicable collection point for the recycling of electrical and electronic equipment.
Disposal must be carried out in accordance with local environmental regulations for
waste disposal.
For more detailed information about treatment, recovery and recycling of this prod-
uct, please contact your local city office, your household waste disposal service or the
shop where you purchased the product.

Operating Manual Version 7.3-03 of 28.07.20
3
Origin: RITM OKB ZAO, 99, Petrovskaya, Taganrog, 347900, Russia.
Model: RITMSCENAR Super Pro v.2.
Classification: Type of protection against electric shock –Internally
powered equipment (4 batteries each of 1.5 V) Applied parts –Type BF.
Waterproofing: No special protection against liquid ingress provided
(IPX0).
Cleaning & Disinfecting: Wipe electrode area with a cotton swab damp-
ened with 3 % hydrogen peroxide solution with the addition of 0.5 % so-
lution of an approved cleaning liquid. Allow to dry up thoroughly before
use. Clean case of device and add-on electrodes with a damp (not soak-
ing) cloth and mild soap solution. Allow to dry before use.
Do not sterilize SCENAR device and add-on electrodes.
Do not expose any part of SCENAR device and add-on electrodes to
chemical solvents or harsh cleaning fluids. Follow cleaning instructions
in this manual.
Clinical environment: NOT suitable for use in the presence of flamma-
ble anaesthetic mixtures with air, oxygen or nitrous oxides.
Add-on electrodes: Only add-on electrodes supplied by the manufacturer
can be used. They are suitably for electromagnetic emitting suppressing.
DO NOT DISASSEMBLE the device –this can be done by special
service personnel only.
Batteries: Remove batteries from the device if not in use for an extended
period. Connect correctly. DO NOT TRY TO RECHARGE disposable
batteries! Dispose of used batteries responsibly. Use good quality, within-
date long-life, 1.5 V ALKALINE Type LR03 (AAA) batteries.
Note
Total batteries removal should be used during storage and trans-
portation to avoid battery drain.
The device should NOT be operated with the battery cover re-
moved, as this exposes the operator to battery circuits in contra-
vention of the Safety Regulations.

Operating Manual Version 7.3-03 of 28.07.20
4
MARKS AND SYMBOLS ON THE DEVICE CASE
THIS CE SYMBOL CERTIFIES THAT
THE PRODUCT COMPLIES WITH
THE ESSENTIAL REQUIREMENTS
OF THE MEDICAL DEVICE
DIRECTIVE
Notified Body No.2265
3EC International a.s., Hraničná 18,
Bratislava, 82105, Slovakia
APPLIED PARTS –TYPE BF
MANUFACTURER combined with
DATE OF MANUFACTURE
SN-XXXX
SERIAL NUMBER
CONSULT INSTRUCTIONS FOR
USE
AUTHORISED REPRESENTATIVE
IN THE EUROPEAN COMMUNITY

Operating Manual Version 7.3-03 of 28.07.20
5
CONTENTS
MARKS AND SYMBOLS ON THE DEVICE CASE..................................4
1 PURPOSE ..............................................................................................6
2 SPECIFICATIONS..................................................................................6
3 DELIVERY SET....................................................................................12
4 DEVICE COMPONENTS AND CONTROLS .......................................12
5 PREPARING DEVICE FOR OPERATION...........................................14
6 USING THE DEVICE............................................................................15
6.1 GENERAL INSTRUCTIONS ............................................................. 15
6.2 MAIN MENU ....................................................................................... 18
6.2.1 Setting the Dose (Dose)..........................................................18
6.2.2 Setting the Amplitude Modulation (AM). .................................23
6.2.3 Setting the Frequency Modulation (FM). ................................23
6.2.4 Setting the Damping mode (Dmp)..........................................24
6.2.5 Setting the Frequency (F) .......................................................24
6.2.6 Setting the Intensity (Int).........................................................24
6.2.7 Setting the Stimulus Gap in a Burst (Gap)..............................24
6.2.8 Switching Off the Device.........................................................26
6.2.9 Switching the Device to the Standby Mode ............................26
6.3 SERVICE MENU ................................................................................ 26
6.3.1 Setting the Automatic Turn-Off Time (AOff)............................27
6.3.2 Setting the Backlight Time (Lght)............................................27
6.3.3 Setting the Screen Contrast (Cont).........................................28
6.3.4 Setting the Default Screen Direction (Save Scr) ....................28
6.3.5 Setting the Language (Lng) ....................................................28
6.3.6 Setting the Sound Volume (Snd)............................................28
6.3.7 Saving the Settings (Write).....................................................28
6.3.8 Reading the Settings (Read)...................................................28
7 MAINTENANCE SERVICE ..................................................................29
8 TROUBLESHOOTING .........................................................................29
9 WARRANTY.........................................................................................31
10 TRANSPORTATION AND STORAGE ..............................................32
ANNEX 1 .................................................................................................33

Operating Manual Version 7.3-03 of 28.07.20
6
1 PURPOSE
RITMSCENAR Super Pro v.2 transdermal
electroneurostimulator –(hereinafter –the device or SCENAR) –is
intended for delivering general therapeutic non-invasive treatment to the
physiological systems of the body via human skin areas in order to treat
various pathologies.
The device is intended to treat and rehabilitate people and can be
used by medical professionals in medical-prophylactic institutions, hospi-
tals, emergency care units as well as at home according to the doctor’s
prescription.
The device should be used under temperatures between 10 C and
35 C with relative humidity not to exceed 80 % at a temperature of 25 C.
Potential risk from the device usage refers to Class IIa (2a)
DIRECTIVE 93/42/EEC (GOST R 31508).
The device complies with the standards EN 60601-1 (GOST R
50267.0) and EN 60601-2-10 (GOST R 50267.10) for internally powered
equipment, type BF, which classifies it as a safe device for personal use.
2 SPECIFICATIONS
2.1 Supply voltage –from 4 up to 6.4 V (four 1.5 V alkaline batteries).
2.2 Maximum supply current –not greater than 650 mA.
2.3 At a load as shown in Fig.1 SCENAR provides:
2.3.1 two-phase stimuli without a DC-component (see Fig.2) with a
waveform depending on the skin impedance under the electrode
(see Fig.3 through 5) generated at a fixed frequency that can be
controlled within 15 to 350 Hz ± 5 %.
2.3.2 control of the stimulus’ 1st phase duration (see Fig.2) within
(4 ±2) to (500 ±50) µsec, and the amplitude of the first pulse of
the stimulus 2nd phase at L1 load as shown in Fig.1 varies from
(1.7…2.5) V to (100…150) V;

Operating Manual Version 7.3-03 of 28.07.20
7
C1 K73-11-630V-2200 pF ±10 %
С2, С3 K73-11-250V-0.033 μF ±10 %
R1 1/4W 11 kΩ±5 %
R2, R3 1/4W 91 kΩ±5 %
R4 1/4W 560 Ω ±5 %
M1…M3 are measuring points
Fig.1
1st Ph –stimulus’ 1st phase duration
2dPh –stimulus’ 2nd phase duration
Ua–stimulus’ 2nd phase 1st pulse
amplitude Fig.2
Load L1; S2 –‘Off’,
load capacity –33 nF
Fig.3
“0”
“L1”
“L2”
C1 C2 C3
R1 R2 R3 R4
S1
T
M1
S2 M3
M2

Operating Manual Version 7.3-03 of 28.07.20
8
Load L1, S2 –‘On’,
load capacity –66 nF
Fig.4
Load L2,
load capacity –2.2 nF
Fig.5
2.3.3 Amplitude modulation (see Fig.6) with the following settings:
pause time: (1.0 ±0.5) sec;
stimulus burst time:
in the 1:1 mode –(1.0 ±0.5) sec;
in the 2:1 mode –(2.0 ±0.5) sec;
in the 3:1 mode –(3.0 ±0.5) sec;
in the 4:1 mode –(4.0 ±0.5) sec;
in the 5:1 mode –(5.0 ±0.5) sec.
Umin –minimum amplitude
Us–set amplitude
tp–pause time
tb–stimulus burst time
Fig.6
2.3.4 BEE mode –generation of a single stimulus with the first
pulse of the 2nd phase having the highest amplitude;

Operating Manual Version 7.3-03 of 28.07.20
9
Fig.7
2.3.5 Frequency modulation (variable frequency) with the follow-
ing settings:
variation range –30 to 120 Hz ±5 %;
variation period –(7 ±2) sec;
2.3.6 Damping modes (change of influencing stimulus’ initial
shape):
Dmp Off (no damping) (see Fig.8);
Dmp Sc1 (weak damping) (see Fig.9);
Dmp Sc2 (moderate damping) (see Fig.10);
Dmp Sc3 (strong damping) (see Fig.11);
Dmp Sc4 (maximum damping) (see Fig.12);
Dmp Var –variable damping with the following settings:
◊damping variation range: from Dmp Off to Dmp
Sc4 and back;
◊period of damping variation: (7.5 ±2.0) sec.
Fig.8
Fig.9

Operating Manual Version 7.3-03 of 28.07.20
10
Fig.10
Fig.11
Fig.12
2.3.7 Generation of stimulus bursts.The number of stimuli in a burst –
intensity –is controlled within 1 to 8 at a step of 1, and the pause be-
tween stimuli in a burst –(between the end of the 1st phase of the
current stimulus and the beginning of the 1st phase of the following
stimulus, called Gap) –from (200 ±10) to (1600 ±100) µsec;
2.3.8 Combined modulation (swing) modes:
2.3.8.1 Sw1 with the following settings:
frequency modulation according to p.2.3.5;
variable damping according to p.2.3.6;
intensity 3;
gap continuously changes from (200 10) to
(1600 100) µsec and back.

Operating Manual Version 7.3-03 of 28.07.20
11
b1, b2, b3 –beginning of the 1st phase of stimuli
e1, e2, e3 –end of the 1st phase of stimuli
Fig.13
2.3.8.2 Sw2 with the following settings:
frequency modulation according to p.2.3.5;
variable damping according to p.2.3.6;
intensity 3;
gap randomly changes from (200 10) to (500 25) µsec.
2.3.8.3 Sw3 with the following settings:
frequency modulation according to p.2.3.5;
variable damping according to p.2.3.6;
intensity 3;
gap randomly changes from (200 10) to (1460 100) µsec.
2.3.8.4 Sw4 with the following settings:
frequency modulation according to p.2.3.5;
variable damping according to p.2.3.6;
intensity randomly changes from 1 to 4;
gap continuously changes from (200 10) to
(1600 100) µsec and back.
2.3.9 (Optional) Automatic modulation with the following settings:
intensity automatically changes from 1 to 7;
gap automatically changes from (200 10) to
(1600 100) µsec;
frequency:
ois set manually from 15.3 to 90.7 Hz;
or
ois set automatically from 60 to 350 Hz.

Operating Manual Version 7.3-03 of 28.07.20
12
2.4 Device’s weight – not greater than 0.4 kg.
2.5 Overall dimensions –not greater than 190 70 40 mm.
2.6 Average nonfailure operating time –not less than 1000 hours.
2.7 Average service life –not less than 5 years.
3 DELIVERY SET
SCENAR complete delivery sets are given in Table 1:
Table 1
Article
Quantity
(units)
RITMSCENAR Super Pro v.2
Local electrode
1
1
Alkaline battery AAA type
4
Cover
1
Operating Manual
1
Instruction for Use
1
Note:
1) On the customer’s request, the electroneurostimulators can be
completed with the following add-on electrodes:
–Face electrode
–Comb electrode
–Point electrode
–Special Snail electrode
–Bent point electrode
–Double facial Pawns electrode
–Double cosmetic electrode
–Double ophthalmic Goggles electrode
–Double facial Stamps electrode
–Single ophthalmic Monocle electrode
–Special double Pencils electrode
–Large comb electrode
–Multi-purpose zonal electrode
2) Add-on electrodes listed in p.1) can be purchased on the custom-
er’s request at extra cost.

Operating Manual Version 7.3-03 of 28.07.20
13
4 DEVICE COMPONENTS AND CONTROLS
4.1 The device is illustrated in Fig.14. The device has an upper cover 1
with a screen 8, case with a built-in electrode 9 and a battery cover 2. All
components except for the batteries are located on the printed circuit
board inside the device’s case.
4.2 The LCD screen 8 displays the results of measuring, settings and
device’s state.
Fig.14
4.3 The device has the following controls on the upper side 1 of its case:
button 3 (–) is used to decrease the energy strength or value
of a selected parameter;
button 4 ( ) is used to select parameters and exit the standby
mode;
button 5 (+) is used to increase the energy strength or value
of a selected parameter;
button 10 () –is used to rotate the picture on the screen.
4.4 The lateral surface of the case has a switch button 6 to switch
on/off the device and plug 7 for connecting add-on electrodes.
4.5 The device is powered from 4 alkaline batteries (AAA Type).

Operating Manual Version 7.3-03 of 28.07.20
14
5 PREPARING DEVICE FOR OPERATION
5.1 Before every procedure disinfect electrodes with a cotton wad
wetted in a 3 % hydrogen peroxide solution with the addition of 0.5 %
solution of an approved cleaning liquid. Allow to dry up thoroughly be-
fore use. Clean case of device and add-on electrodes with a damp (not
soaking) cloth and mild soap solution. Allow to dry before use.
5.2 Open the battery compartment cover and
install the batteries. Switch the device on using
the switch button on the lateral panel. A message
containing the information about the device’s
name, version and its software release date
(Fig.15) will be displayed for 2 seconds and a
beep will sound.
5.3 Then the device switches to the state that
displays basic parameters (hereinafter called ‘B’
state). So, the basic parameters of stimulation
will be displayed (Fig.16).
Fig.15
The first line contains the timer, indica-
tor of the device’s contact with the patient’s
skin (●–contact, ○–no contact), and a
battery charge indicator. The timer is reset
upon pressing any button, and when au-
tomatic turn-off is enabled, the timer is
reset on contacting the skin as well.
5.4 Press the button to switch to
the Menu state (Fig.17). Press the
button at an interval of no longer than
2 seconds to make sure that the highlight-
ing switches cyclically.
Then wait until the device switches to
the ‘B’ state (this will happen in
2 seconds after the latest pressing of the
button ), then press the –button once.
A long (1 sec) beep will sound.
Fig.16
Fig.17
Press and hold the button +. The number in the right part of the top
line will be changing from 1 to 250, and then a long beep will sound.

Operating Manual Version 7.3-03 of 28.07.20
15
If you performed all the operations mentioned above in this item and
the device works as described, the device is ready to be used for therapy.
Otherwise, refer to the Chapter 8 (Troubleshooting).
5.5 Switch off the device using the switch button.
6 USING THE DEVICE
6.1 GENERAL INSTRUCTIONS
6.1.1 When manipulating the device, observe the indications on the
screen and beeping.
6.1.2 A chart of the device’s basic states is illustrated in the Fig.18
(dotted lines mean that the Dose is on).
Fig.18
After being switched on, the device is always in the ‘B’ state. In
the ‘B’ state the device will stimulate at the settings displayed on the
screen. Use the +and –buttons to control the energy strength of stimu-
lation. Use the button to switch the device to the Menu state.
In the Menu state you can set all parameters except for the energy
strength. For detailed description of these parameters see the items 6.2.1-
6.2.9. A current parameter is highlighted inversely. Use the +and –but-
tons to change the current parameter’s value. Use the button to move
down the menu, use the button to move up the menu. If no buttons are

Operating Manual Version 7.3-03 of 28.07.20
16
pressed within 2 seconds, the device will switch to the ‘B’ state, or, if
any dosing is on, the device will switch to the Dose state.
To activate the dosing, set the Dose parameter to 1, 2, 3, 4, or 5
in the menu.
In this state pressing the +and –buttons switches the device to the
‘B’ state to control the energy value (the device will return to the Dose
state if no buttons are pressed within 2 seconds).
To switch the device to the Menu state, use the button .
6.1.3 The device supports 4 screen orientations: 2 landscape modes
(Fig.19a) and 2 portrait modes (Fig.19b). To change the screen orienta-
tion, press the button in the ‘B’ state or in the Dose state.
a
b
Fig.19
6.1.4 There is a battery indicator in the top line of the screen. The
degree of its infill shows the battery’s charge. If the indicator is blank,
remove all 4 batteries and install 4 new ones.
6.1.5 When switching on the device, the following parameters are set:
the energy strength of stimulus is set to the minimum set-
ting (1);
dosing is switched off (Off);
amplitude modulation is switched off (Off);
frequency modulation is switched off (Off);
stimulus frequency is set to 90.7 Hz;
damping is switched off (Off);
intensity is set to the minimum setting (1);
gap between stimuli in a burst is set to the minimum value (10).

Operating Manual Version 7.3-03 of 28.07.20
17
Attention!
To avoid painful sensations when treating the most sensi-
tive areas on the patient’s body, it is recommended that the
energy strength is decreased to minimum before the treat-
ment by pressing and holding the –button until a long
beep sounds).
6.1.6 Apply the electrode on the skin on the zone to be treated. The ●
symbol will be displayed on the screen indicating that the electrode is
contacting the skin. Make sure that patient isn’t experiencing unpleasant
sensations. Press and hold down the +button till the first sensation like
pricking, light burning or vibrations appear. They shall be sensed as com-
fortable and not painful, but must be felt on the skin.
Attention!
When using add-on electrodes, connect electrode cable
only to the switched-off device, in order to prevent the de-
vice damage and to avoid painful sensations!
6.1.7 To start therapy, set the required mode. Then put the electrode
on the skin area to be treated (see the Instruction for Use provided with
your device).
6.1.8 To treat auricular zones and acupuncture points, connect your
add-on point electrode to the appropriate jack. Make sure of the contact
by touching the skin with the electrode. Before every treatment of any
acupuncture point, dip the end of the probe in water to wet the surface.
Stimulation by point electrode that is too small or by any electrodes
that applied incorrectly could result in discomfort.
Note
When using add-on electrodes, especially point and comb, the
dosing mode can be out-of-work even in case of good contact with
skin and even if the patient feels the stimulation. It is not a fault.
In the ‘B’ state the +and –buttons are used to control the energy
strength within the range from 1 to 250 units. To control the energy
strength you can either press the buttons ‘step by step’ or press and hold
the appropriate button (this way is faster, 16 steps per second). When the
energy strength reaches the value that cannot be further in-
creased/decreased, the device will indicate it with a long beep.
6.1.9 If the automatic turn-off is enabled (see p. 6.3.1), and if there is
no contact with the skin for 30 seconds, the device will automatically
switch to the standby mode. This will be accompanied by a beep, text
message and screen darkening. To resume SCENAR operation, press the
button.

Operating Manual Version 7.3-03 of 28.07.20
18
6.2 MAIN MENU
6.2.1 Setting the Dose (Dose)
When Dose Off is set (which is indicated by a long beep), the Dos-
ing is off.
Dose 1 (indicated by a short beep) activates the individually-dosed
mode (hereinafter –IDM) with the adaptive integral criterion of deliv-
ered stimulation (Dose) and integral criterion of achieving the null rela-
tive dynamics (Zero).
Dose 2 (indicated by 2 short beeps) activates IDM with the differ-
ential criterion of achieving the null dynamics (Differential Dose).
Dose 3 (indicated by 3 short beeps) activates the Screening
mode (for searching the optimal treatment places).
Dose 4 (indicated by 4 short beeps) is similar to Dose 3 but the
data are displayed in the circle (when the screen image is horizontal).
Optional Dose 3 and/or Dose 4 modes can represent the labile
screening mode data (for searching skin areas with maximal reaction in a
labile mode): current reaction to the initial reaction ratio in the audio-
visual mode.
Dose 5 (indicated by a dual-tone beep) is used to carry out
electropunctural diagnostics in 24 points (6 points on each extremity)
with an add-on local electrode.
When switching between dose modes, damping switches off au-
tomatically.
After Dose 1 or Dose 2 has been selected, if no buttons are pressed
within 2 seconds, the device will switch to the Dose state (Fig.20).
a
b
Fig.20

Operating Manual Version 7.3-03 of 28.07.20
19
Once the device detects that the electrode is contacting the skin, the
device will give a short low pitch signal (○will change to ●in the top
line), and in a second it will give a short high pitch signal and the screen
will display (both digitally and graphically) the results measured over the
first second (the diagram will show current (ongoing) reaction change
with time).
After 1 second more, measurement results for the 2nd second will be
displayed on the screen. Then, current results of measuring (time, reac-
tion, rate of reaction change, shape factor) illustrated in the Fig.21 will be
updated every second, and the diagram will be filled up. It will continue
this way until either Dose or Zero is achieved.
Timer
Initial reaction
Initial shape factor
‘Dose’
Current shape factor
Reaction rate
Reaction and shape
rate chart
‘Zero’
‘Dose’ ‘Zero’
Current reaction
Fig.21
In Dose 1,until Dose is delivered, the device emits beeps indicating
the time elapsed from the moment the electrode contacted the skin. At the
10th second the device emits one short beep, at the 17th sec –two beeps, at
the 24th sec –three beeps, and so on –n beeps at the (3+7*n) second.
When the Dose is reached it is simultaneously indicated by:
2-second dual-tone beep and;
the *symbol displayed along with the values of the reaction and
shape factor registered at a point of the Dose (in red).
When the Zero is reached it is simultaneously indicated by:
series of low pitch sounds during 2 seconds;
the ○symbol displayed along with the values of the reaction and
shape factor registered at a point of the Zero (in blue).

Operating Manual Version 7.3-03 of 28.07.20
20
Once you take the device up from the skin, ●will change to ○in the
top line, and all other information will stay the same on the screen.
In the Dose state, pressing any button resets the timer and
measurements. Moreover, touching the skin with the electrode also
resets the timer.
When switching on Dose 3, Dose 4, and Dose 5, all modula-
tions and damping are switched off forcedly.
Data displayed on the screen at Dose 3 are shown in Fig.22, and
those for Dose 4 are illustrated in Fig.23. In these modes after 1-second
stimulation a short beep will sound, the energy strength will decrease to
the minimal level, and the device will be waiting for the electrode to be
taken off from the skin. Once the electrode is taken off, the previously set
value of the energy is restored.
Successively applying the device on the points to be treated, you can
get up to 8 results of initial measurements on the screen.
Fig.22
Every next measuring, its results are displayed in the next line. For
Dose 3 the results of the 8th measuring will be displayed in the 8th line,
and those of the 9th measuring will be displayed again in the in the 8th line.
So, all previous lines will as if move one line upwards. So, in the Dose 3
screening mode, the screen displays the results of initial measurements for
the latest 8 points.In Dose 4 horizontal and vertical data display are dif-
ferent.
Table of contents
Other RITM OKB ZAO Personal Care Product manuals