800987 Rev H (Side 1) Printed on 25 March 2021
Salter AIRE
Elite
™
Compressor
Instruction Manual
Part No: 8350-1 / 8352-1 / 8353-1
Thank you for selecting the Salter AIRE
Elite
Compressor. Salter Labs is an innovative, industry-
leading manufacturer of respiratory
care devices. Please contact your local Salter Labs
dealer for information about additional products.
SAVE THESE INSTRUCTIONS.
READ ALL INSTRUCTIONS BEFORE USE.
Contents
Important Safeguards………………………………………… 1
Introduction…………………………………………………….. 2
Product Description…………………………………………… 3
Operation………………………………………………………… 4
Cleaning………………………………………………………….. 5
Storage…………………………………………………………… 6
Maintenance……………………………………………………. 7
Expected Service Life ………………………………………... 8
Troubleshooting……………………………………………….. 9
Specifications………………………………………………….. 10
Accessories……………………………………………………... 11
Appendix A…………………………………………………….. 12
1. Important Safeguards
NOTE, CAUTION, WARNING, AND SYMBOLS:
Important information is highlighted by using the following:
NOTE Indicates information that user should pay special
attention to.
CAUTION Indicates correct operating or maintenance
procedures in order to prevent damage to or destruction of
the equipment or other property.
WARNING Indicates potential danger that requires correct
procedures or practices in order to prevent personal injury
.
Symbols:
Off, disconnection from the mains
On, connection to the mains
~Alternating Current (AC)
IP2X Protected against solid foreign objects having a
diameter of 12.5 mm and greater. No protection
against vertically falling water drops. Keep dry!
Attention
Class II
Refer to instruction manual/ booklet
“BF” symbol, indicate this product is according to
the degree of protecting against electric shock for
Temperature limitation
Disposal of Electrical & Electronic Equipment
(WEEE): This product should be handed over to an
applicable collection point for the recycling of
electrical and electronic equipment. For more
detailed information about the recycling of this
product, please contact your local city office,
household waste disposal service or the retail
store where you purchased this product.
CAUTION U.S. Federal Law restricts this device to sale by or
on the order of a physician.
WARNING To reduce the risk of burns, electrocution, fire or
injury to persons:
1. Always unplug this product immediately after using.
2. Do not use while bathing, showering, dish washing, or
close to water sources of any kind.
3. Do not place or store product where it can fall or be
pulled into a tub or sink.
4. Do not place in or drop into water or other liquid.
5. Do not reach for a product that has fallen into water.
Unplug immediately.
6. This product should never be left unattended when
plugged in.
7. Close supervision is necessary when this product is used
by, on or near children or invalids. Choking accident may
result from a child swallowing a small part that has
become detached from the device or its accessories.
8. Use this product only for its intended use as described
in this manual. Use this product only under doctor’s
direction. Do not use attachments not recommended
by the manufacturer.
9. Never operate this product if a) it has a damaged cord or
plug, b) it is not working properly, c) it has been dropped
or damaged, d) it has been dropped into water. Return
the product to a specified service center for
examination
and repair.
10. Keep the cord away from heated surfaces.
11. Never block the air openings of this product or allow
objects to fall or be inserted into the air vent openings
or place it on a soft surface such as bed or couch, where
the air openings may be blocked.
12. Never use while sleeping or feeling drowsy.
13. Never drop or insert any object into any opening or
hose.
14. No modification of this equipment is allowed.
15. Do not modify this equipment without authorization of
the manufacturer.
16. If this equipment is modified, appropriate inspection
and testing must be conducted to ensure continued safe
use of the equipment.
17. Do not use in outdoors or operate where aerosol (spray)
products are being used or where oxygen is being
administered in a closed environment such as an
oxygen reservoir.
18. Do not wrap the power cord around the compressor
(main unit).
19. Disconnect the power plug by pulling the plug, not by
pulling on the compressor (main unit), or the cord.
20. If the power cord or plug becomes frayed or otherwise
damaged, do not use.
21. Do not place heavy objects on the power cord, or bend
and pull the cord harder than necessary. These actions
could cause an electric shock or fire.
22. Potential allergic reactions to accessible materials used
in the Compressor Nebulizer equipment. If any signs of
allergic reaction or hypersensitivity happen, stop the
treatment immediately, and notify the doctor or nurse.
23. Potential contact injuries for patients used in the
Compressor Nebulizer equipment. If any contact injuries
happen, stop the treatment, and notify the doctor
or nurse.
24. The device enclosure may overheat, users do not touch
more than 10 seconds.
WARNING EMC Statement
This equipment has been tested and found to comply with
the limits for medical devices to the IEC 60601-1:2014. These
limits are designed to provide reasonable protection against
harmful interference in a typical medical installation. This
equipment generates uses and can radiate radio frequency
energy and, if not installed and used in accordance with the
instructions, may cause harmful interference to other
devices in the vicinity. However, there is no guarantee that
interference will not occur in a particular installation. If this
equipment does cause harmful interference to other
devices, which can be determined by turning the equipment
off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
Reorient or relocate the receiving device.
Increase the separation between the equipment.
Connect the equipment into an outlet on a circuit
different from that to which the other device(s) are
connected.
Consult the manufacturer or field service technician
for help.
CAUTION If there is a possibility of electro-magnetic
interference with mobile phones, please increase the
distance (3.3m) between devices or turn off the mobile
phone.
2. Introduction
2.1 Intended Use
The Salter AIRE
Elite
Compressor System is intended to
provide a source of compressed air for aerosol therapy. It is
used in conjunction with a jet (pneumatic) nebulizer to
produce medicated aerosols for inhalation by pediatric and
adult patients with respiratory symptoms.
CAUTION Aerosolize liquid medication except Pentamidine
for inhalation by the patients. Indications for therapy
include asthma, chronic bronchitis, infection of the upper
respiratory tract, chronic obstructive pulmonary disease
(COPD) and other respiratory disorders in accordance with a
medical doctor’s prescription. Except the usage mentioned
above, please do not use this product for any other purpose.
This device can be used with adults or pediatric patients
under physician’s prescription.
2.2 Safety Precaution Instruction
When using this electrical product, especially when children
are present, one should always follow basic safety
precautions. Do not install, maintain or operate this
equipment without reading, understanding and following
the proper Salter AIRE
Elite
Compressor System instruction
manual, otherwise injury or damage may result.
For 120V only-
This appliance has a polarized plug (one blade
is wider than the other). To reduce the risk of electric shock,
this plug is intended to fit into a polarized outlet only one
way. If the plug does not fit fully into the outlet, reverse the
plug. If it still does not fit, contact a qualified electrician.
Do not modify the plug in any way.
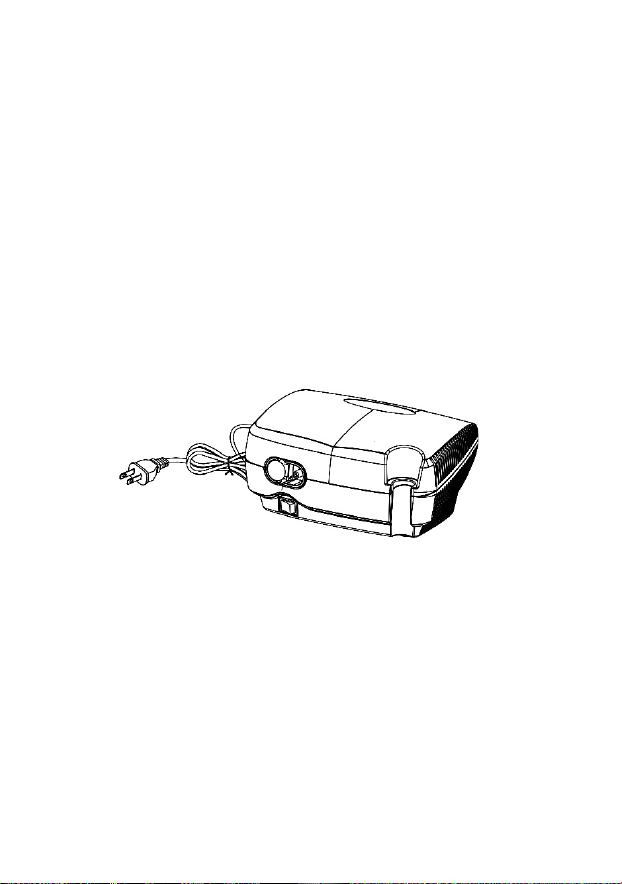
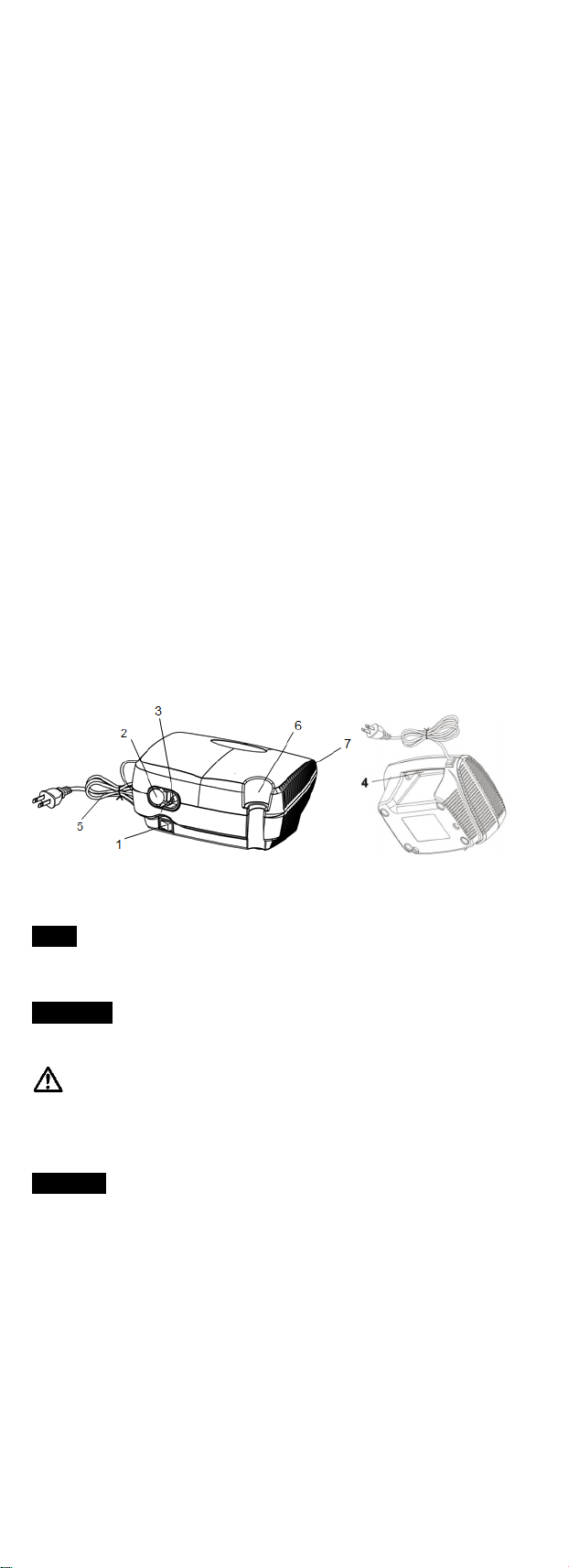
3. Product Description
1. Power Switch 2. Filter Cap (disposable filter
inside)
3. Air-Outlet Connector 4. Integrated Carrying Handle
5. Power Cord 6. Nebulizer Cup Holder
7. Cooling Air Openings
4. Operation
NOTE Before initial operation, the nebulizer cup assembly
should be cleaned following instructions described in the
“Cleaning” section.
WARNING Before connecting the power cord, make sure the
I/O (ON/OFF) switch is in the O (OFF) position.
The plug is also served to disconnect the device. Do not
position the equipment so that it is difficult to operate
the disconnecting device.
4.1 Daily Use Operation
CAUTION The Salter AIRE Elite Compressor System is
designed for intermittent use only. Do not operate it
continuously for more than 30 minutes for a single use
without turning it off and following a cooling period for least
30 minutes.
1. Before each use inspect the Salter AIRE
Elite
Compressor and nebulizer cup assembly for damage or
wear, replace as needed.
2. Place the Salter AIRE
Elite
Compressor on a table or
other flat stable surface. Be sure you can easily reach
the controls when seated. Do not use this device on the
floor.
3. With the power switch in the O (OFF) position, plug the
power cord into an appropriate electrical wall outlet.
4. Connect one end of the tubing to the compressor air-