Pilot²
- 10 -
Table of contents......................................................................................................................... Page
1.1. Symbols used ........................................................................................................................... 11
1.2.Intended use ............................................................................................................................. 13
1.3. Device Description ................................................................................................................... 13
1.3.1. Apex locator ................................................................................................................ 13
1.3.2.Motor ........................................................................................................................... 13
1.3.3. DownPack hand-piece with heating tip ........................................................................ 13
1.3.4. BackFill gun................................................................................................................. 13
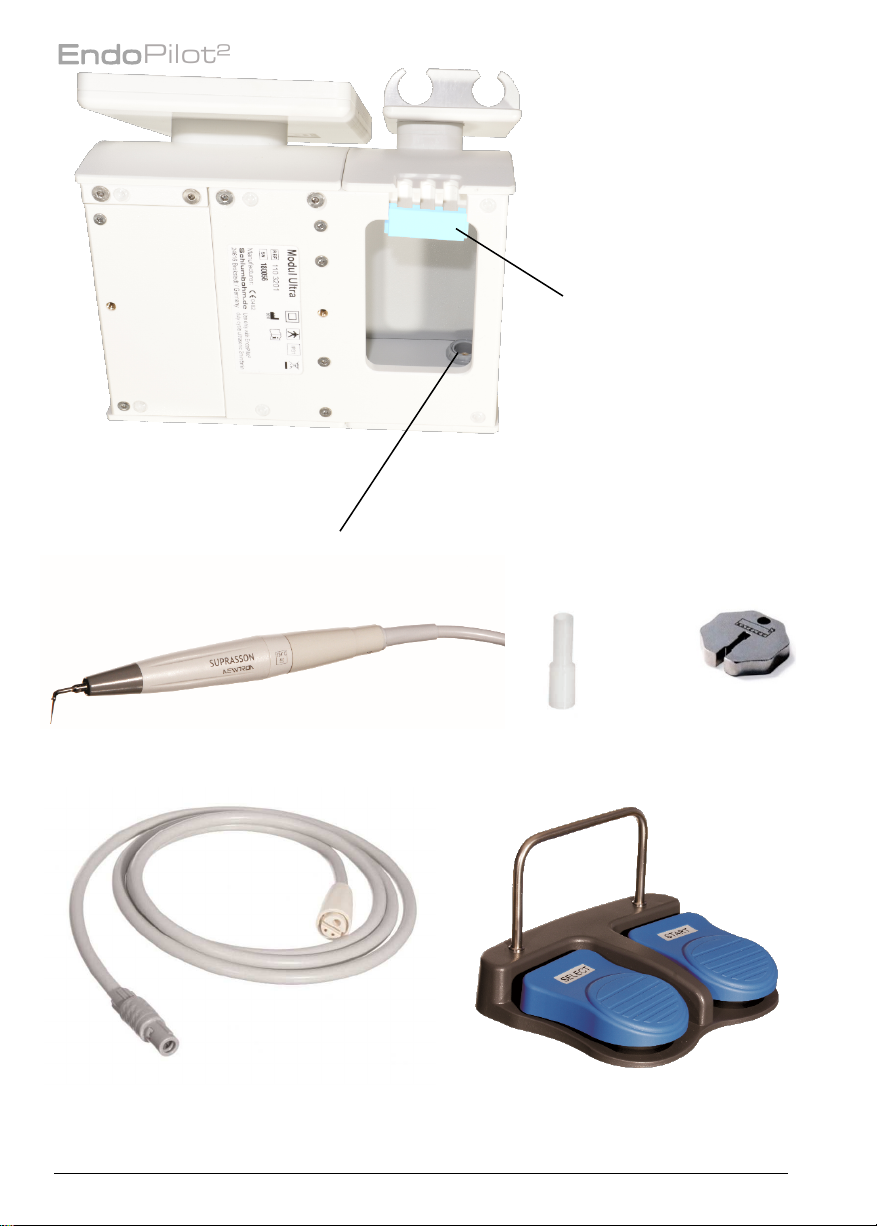
1.3.5. Ultrasonic handpiece*.................................................................................................. 13
1.4. General precautions................................................................................................................. 14
1.4.1. Contraindications......................................................................................................... 14
1.4.2. Operating instructions.................................................................................................. 14
2. First steps.................................................................................................................... 16
2.1. Assembly ................................................................................................................................... 16
2.2. Holders for the handpieces ..................................................................................................... 17
2.3. Connection ................................................................................................................................ 17
2.4. Touch display ............................................................................................................................ 18
2.5. Foot switch ................................................................................................................................ 18
2.6. Charging, switching-on, standby mode, switching-off ......................................................... 19
2.7. Preparation of the root canal - motor and contra-angle....................................................... 19
2.8. Filling technique - DownPack (D-Pack)................................................................................. 20
2.9. Filling technique - BackFill....................................................................................................... 21
3. Manual apex length determination ............................................................................... 22
3.1. Tips for length determination................................................................................................... 23
4. Motor system............................................................................................................... 24
4.1. Favorites .................................................................................................................................... 24
4.2. Selection of the file systems ................................................................................................... 24
4.3. Preparation................................................................................................................................ 25
4.4. MyFile file system..................................................................................................................... 25
4.5. Setup motor............................................................................................................................... 26
4.5.1. File data ...................................................................................................................... 26
4.5.2. Reciprocal function ...................................................................................................... 27
4.5.3. Apex functions during motor operation......................................................................... 28
4.5.4. Calibrate...................................................................................................................... 29
5. Obturation ................................................................................................................... 30
5.1. DownPack ................................................................................................................................. 30
5.2. BackFill ...................................................................................................................................... 30
6. Ultrasonic function*...................................................................................................... 31
6.1. Operating instructions* ............................................................................................................ 31
6.2. Setting the ultrasonic power output* ...................................................................................... 32
6.3. Ultrasonic instrument selection* ............................................................................................. 32
6.4. Setting the run time*................................................................................................................. 32
7. Software release and updates ..................................................................................... 33
8. Brightness / Volume..................................................................................................... 33
9. Setting the language.................................................................................................... 33
10. Auto-off time................................................................................................................ 33
11. Service information / Bluetooth .................................................................................... 33
12. Maintenance, transport and disposal ........................................................................... 34
12.1. Periodical tests ......................................................................................................................... 34
12.2. Maintenance.............................................................................................................................. 35
12.3. Transport ................................................................................................................................... 35
12.4. Disposal..................................................................................................................................... 36
13. Troubleshooting........................................................................................................... 37
14. Error messages ........................................................................................................... 39
15. Warranty / Liability ....................................................................................................... 39
16. Technical Data............................................................................................................. 40
17. EMC manufacturer's declaration.................................................................................. 41
18. Cleaning, disinfection sterilization (Processing) ......................................................... 44