SOMNOmedics SOMNOtouch RESP User manual

SOMNOmedics GmbH
Am Sonnenstuhl 63 –97236 Randersacker –Germany
Phone: (+49) 931 / 35 90 94-0 –Fax: (+49) 931 / 35 90 94-49
I
NSTRUCTION MANUAL
SOMNOtouchTM RESP
Caution: Federal law restricts this device to sale by or on the order of a physician.

- 2 -
Manufacturer:
SOMNOmedics GmbH
Am Sonnenstuhl 63 –97236 Randersacker –Germany
Phone: (+49) 931 / 35 90 94 - 0 –Fax: (+49) 931 / 35 90 94 - 49
Rev. 0
29.01.2015
All proper names marked with TM are copyright protected by SOMNOmedics.

- 3 -
Index
1Introduction ..................................................................................................................................... 6
1.1 Intended Use / Indication of Use ................................................................................................ 6
1.2 Overview..................................................................................................................................... 6
1.3 Patients....................................................................................................................................... 7
1.4 About this instruction manual ..................................................................................................... 7
1.5 Explanation of Symbols used in this Manual.............................................................................. 7
2About the SOMNOtouch™ RESP................................................................................................... 8
2.1 Type Label.................................................................................................................................. 8
2.2 Elements of keyboard............................................................................................................... 10
2.3 Configuration ............................................................................................................................ 11
3Safety instructions........................................................................................................................ 12
4Installing the DOMINO light Software ......................................................................................... 14
5Manual Docking Station Driver Installation................................................................................ 19
5.1 Windows 7................................................................................................................................ 19
6Operating Instructions.................................................................................................................. 23
6.1 Initialising the SOMNOtouch™ RESP ...................................................................................... 23
6.1.1 Easystart............................................................................................................................ 24
6.1.2 Advanced Mode................................................................................................................. 25
6.1.3 Starting the SOMNOtouch™ RESP at the display ............................................................ 28
6.1.4 Programmed start of the SOMNOtouch™ RESP .............................................................. 30
6.2 Attaching the Sensors .............................................................................................................. 31
Display during recording .................................................................................................................... 33
6.3 Settings..................................................................................................................................... 36
6.4 Manual abortion of the recording.............................................................................................. 38
6.5 Data Transfer from SOMNOtouch™ RESP to PC ................................................................... 39
6.6 Open a Recorded Measurement.............................................................................................. 40
6.7 Analysis .................................................................................................................................... 43
6.8 Open the Report....................................................................................................................... 44
7DOMINO light Software................................................................................................................. 51
7.1 System Requirements .............................................................................................................. 51
7.2 DOMINO light Panel................................................................................................................. 52
7.3 Global Preferences................................................................................................................... 53
7.3.1 Menu –Folders.................................................................................................................. 53
7.3.2 Menu –Channels............................................................................................................... 54
7.3.3 Menu –Analysis................................................................................................................. 55
7.3.3.1 Sleep Wake Analysis ................................................................................................ 56

- 4 -
7.3.3.2 Activity Analysis ........................................................................................................ 57
7.3.3.3 Flow Analysis............................................................................................................ 58
7.3.3.4 Snore Analysis.......................................................................................................... 60
7.3.3.5 Phase Angle Analysis ............................................................................................... 60
7.3.3.6 Position Analysis....................................................................................................... 61
7.3.3.7 Effort Analysis ........................................................................................................... 61
7.3.3.8 SpO2 Analysis............................................................................................................ 62
7.3.3.9 CPAP Analysis.......................................................................................................... 63
7.3.4 Menu - Analysis Channels................................................................................................. 63
7.3.5 Menu –Filter...................................................................................................................... 64
7.3.6 Menu - Keys....................................................................................................................... 64
7.3.7 Menu - Markers.................................................................................................................. 65
7.3.8 Menu –Area Definition ...................................................................................................... 65
7.3.9 Menu –User Data.............................................................................................................. 66
7.3.10 Menu –Report................................................................................................................. 66
7.3.10.1 Standard Report...................................................................................................... 66
7.3.10.2 User defined Report................................................................................................ 67
7.4 Analysis .................................................................................................................................... 75
7.4.1 Setting Analysis and Channels.......................................................................................... 75
7.4.2 Functions of the Pop-up Window....................................................................................... 76
7.4.2.1 Functions of the Analysis Pop-up Window ............................................................... 76
7.4.2.2 Function of the Raw Data Pop-up Window............................................................... 77
7.4.3 Layouts for Data Display in Analysis Mode ....................................................................... 78
7.4.4 Inserting Markers............................................................................................................... 78
7.4.5 Deleting, Editing, Adding Markers ..................................................................................... 79
7.4.6 Creating and Editing Samples........................................................................................... 79
7.4.7 The Event List.................................................................................................................... 80
7.4.8 Edit Modes......................................................................................................................... 81
7.4.8.1 Edit Mode.................................................................................................................. 81
7.4.8.2 Quick Edit Mode........................................................................................................ 82
7.4.8.3 Select Edit Mode....................................................................................................... 82
7.4.8.4 Repeat Mode............................................................................................................. 82
7.5 Reports..................................................................................................................................... 83
7.5.1 Reports using the DOMINO light Report Generator.......................................................... 83
7.5.2 Export Result List to MS Excel .......................................................................................... 83
7.6 Form Letters ............................................................................................................................. 84
7.6.1 Creating a Form letter........................................................................................................ 84
7.6.2 Opening a Form Letter....................................................................................................... 85
7.6.3 Save Form Letter in MS Word ........................................................................................... 85
7.7 Data Exchange......................................................................................................................... 86
7.7.1 Data Export as Picture, in RIFF or ASCII Format.............................................................. 86
7.7.2 EDF+ Export ...................................................................................................................... 86
7.8 Archiving................................................................................................................................... 88
7.8.1 Archiving data.................................................................................................................... 88
7.8.2 Archiving database ............................................................................................................ 90
8Troubleshooting............................................................................................................................ 91
9Maintenance of the SOMNOtouch™ RESP................................................................................. 92

- 5 -
9.1 Maintenance rate...................................................................................................................... 92
9.2 Cleaning and Disinfection......................................................................................................... 92
9.3 Use and Maintenance of the Rechargeable Battery ................................................................ 93
9.4 Functional Testing of the integrated Pulse Oximeter ............................................................... 93
10 Service........................................................................................................................................... 94
10.1 Technical specification ........................................................................................................... 94
10.2 Lifetime................................................................................................................................... 95
10.3 Storage................................................................................................................................... 96
10.4 Transport ................................................................................................................................ 96
10.5 Malfunctions ........................................................................................................................... 96
10.6 Warranty................................................................................................................................. 96
10.7 Disposal of Parts and the SOMNOtouchTM Device ................................................................ 96
10.8 Accessories and Spare Parts................................................................................................. 97
10.9 Contact ................................................................................................................................... 98
10.10 Notes .................................................................................................................................... 99
11 Clinical Summary....................................................................................................................... 100
12 EMC declaration ......................................................................................................................... 101
- 6 -
1 Introduction
The SOMNOmedics team would like to thank you for purchasing this product. We are confident that
you will enjoy using the SOMNOtouch™ RESP for many years. The SOMNOtouch™ has been
developed by SOMNOmedics to meet the highest quality control standards available.
Caution: Federal law restricts this device to sale by or on the order of a physician.
In order to obtain the best results from your system, we recommend that you carefully read this
instruction manual before connecting the SOMNOtouch™ RESP to a patient.
Advice and suggestions to improve the functionality of the device and this manual we will be gratefully
accepted.
Warning: Take care in arranging patient and sensor cables to avoid risk of patient entanglement or
strangulation. To minimize the risk of patient strangulation, the sensor and electrode cables
must be carefully placed and secured.
Technical Specifications are subject to change without notice.
The Initialisation of the System and Analysis of the Data must be performed by Trained Operators.
Measurements can be performed in professional healthcare facilities or at the Patients’ home. When
used at the Patients’ home, the Patient should be carefully instructed on how to use and care for the
system.
Please also hand out to the patient a copy of the enclosed Patient Instruction Manual. The initialized
device will automatically start recording the measurement at the pre-set time. The data transfer and
the analysis must be performed by the doctor or qualified physiologist.
The SOMNOtouch™ RESP may not be worn during swimming and showering.
Please refer to the safety instructions in chapter 3.
1.1 Intended Use / Indication of Use
“The SOMNOtouch™ RESP is a portable physiological signal recorder. It is indicated for use in the
recording, displaying, monitoring, printing, and storage of biophysical parameters for the purpose of
assisting in the diagnosis of sleep disorders and sleep related respiratory disorders of adult patients.
The device is intended to be prescribed for use by a physician in the office, sleep laboratory or
patient’s home.
This device is NOT designed to be used in life support situations.”
1.2 Overview
The SOMNOtouch™RESP consists of the following:
1. The recording device (worn on the thorax),
2. the finger probe, which is used to detect SpO2,
3. the external effort sensor,
4. two effort belts (single-use*) to measure thoracic and abdominal expansion,
5. the nasal cannula (single-use*) and the nasal cannula filter (single-use*),
6. the Software DOMINOlight for visualization of the recorded data.
* one sleep session
The SOMNOtouch™RESP typically will be worn at the thorax, attached by a thorax belt. It contains a
sensor to measure the respiratory effort signal of the thorax. The device has an internal

- 7 -
accelerometer, measuring body position and motion activity. It provides a connector to attach a nasal
cannula, which allows the recording of respiratory flow and snore signals with the internal pressure
sensor. Arterial oxygen saturation (SpO2) and pulse rate can be determined via a pulse oximetric
finger sensor. Abdominal respiratory effort is measured with an external sensor, attached with a belt to
the abdomen. This information is stored in the internal memory of the device.
The system provides up to 10 internal channels for data acquisition, Pressure/Flow, Thoracic Effort,
SpO2, Pulse rate, Snoring, Finger Plethysmogram, Body Position, Movement, Patient Marker, CPAP,
and 1 external Abdominal Effort Sensor. The data from all channels can be recorded separately or in
any combination with the other channels.
Information is stored on an internal 512 MB flash memory and can be transferred to a PC via a USB
docking station. The DOMINOlight software retrieves the data from the SOMNOtouch™RESP,
displays and analyzes the data, and can store data for future reference and comparison. The
SOMNOtouch™RESP does not provide automatic diagnosis and is not designed to be used in Life
Support situations.
1.3 Patients
The SOMNOtouch RESP and its components may only be used as follows:
- For adult patients
- Excluded are monitored and intensive care patients
1.4 About this instruction manual
It is essential that you read each paragraph carefully when you see this icon on the left of that
paragraph. This instruction manual is a part of the device and it must be available at all times.
1.5 Explanation of Symbols used in this Manual
Indicates a hint or tip. This Symbol provides assistance with possible problems
when working with the SOMNOtouch™ RESP.
This Warning Symbol indicates potential danger to Patients, Property or Data
Loss.

- 8 -
2 About the SOMNOtouch™ RESP
Fig. 2-1: About the SOMNOtouch™
2.1 Type Label
When unpacking the SOMNOtouch™ RESP, check to make sure that all items are in good condition
and that all accessories correspond to the delivery note. Also compare the Model on the delivery note
with the label on the bottom of your SOMNOtouch™ RESP.
Fig. 2-2: Type Label
Information, Symbols, Icons and Classifications on the Type Label
Read the instruction manual very carefully before you start working with the
SOMNOtouch™.
Direct Current
Protection Class: BF
IP22
This device complies with the IP-Protection-Class “22”.
Serial number
Marker Button
External Signal
Input (AUX)
Interface/Charger
Connector
External Signal
Input (AUX)
Status LED’s
Body Position Sensor
+
Acceleration Sensor
(X,Y & Z direction)
Internal Beeper
On/Off Button
Input for SpO2Sensor
Luer Lock® for Nasal cannula
Internal Effort Sensor

- 9 -
Manufacturer information / address
This device has no SpO2 alarm
Additionally you receive information on the serial number and current firmware of the device when you
press the SOMNOmedics logo in the start display:
Fig. 2-3: Logo information
There you will also find our Hotline number and homepage.
Symbols on the power supply label
Protection Class II
Electronic waste –no domestic garbage
Symbols on the docking station label
IP20
This device complies with the IP-Protection-Class “20”.
Electronic waste –no domestic garbage
USB Interface
Symbols on the effort belt label
Manufacturer information / address
Read the instruction manual very carefully before you start working with the
SOMNOtouch™.
Electronic waste –no domestic garbage
Single-use (which means: usage during one sleep session)
www.somnmedics-diagnostics.com
Firmware: 1.0
Hotline: +49 931 359094 994
SN: TOR 0034

- 10 -
2.2 Elements of keyboard
Picture
Description
Function
Status LED 1
Recording
Green LED
Yellow LED
Idle & Waiting Mode
off
off
Recording Mode
flashes 16x /s
off
Initialisation / data transfer
on
off
Error during initialisation
2s on
+ beep
Status LED 2
Charging
When this LED is Yellow, the battery
is being charged
When this LED gets off while device
is on docking station, the battery is
fully charged
When this LED is off, no charge is
taking place.
Patient Marker
The patient uses the button as a
Marker during the measurement. To
place a marker for denoting an
event, (going to bed, getting up,
taking medication, etc.) the patient
pushes this button.
On/Off
If this button is pressed when the
device is connected to the computer
via the docking station, it will switch
into initialisation mode.
This button switches the device On.
A longer press on the button when in
menu display will turn the device off.
During a measurement the display
can be switched on by pressing this
button.

- 11 -
2.3 Configuration
The configuration includes a SOMNOtouch™ RESP basic device, a thorax effort belt, an SpO2sensor,
one abdomen effort sensor and belt, the nasal cannula with filter, the docking station and power
supply, a USB adapter cable, a carry bag for storing or transporting the SOMNOtouch™ RESP, the
instruction manual and the DOMINO light software for initialization, data transfer and analysis.
Channels:
- Movement by 3 acceleration sensor channels (X,Y & Z direction)
- Body Position
- Patient Marker
- Pulse Rate
- SpO2
- Nasal/Oral Flow
- Snore
- Thoracic Effort
- Abdominal Effort
- CPAP pressure

- 12 -
3 Safety instructions
This instruction manual is regarded as part of the instrument and should always be
kept on hand.
The SOMNOtouch™RESP is NOT designed to be used in a Life Support situation,
surgical rooms, intensive care units, or in emergency vehicles.
The SOMNOtouch™ RESP must be applied only under instruction of a physician.
The SOMNOtouch™ RESP has no audible alarms for monitoring Pulse and SpO2.
The SOMNOtouch™ RESP must be able to measure the pulse properly to obtain
accurate SpO2measurement. Verify that nothing is hindering the pulse
measurement before relying on the SpO2measurement.
Fingernail polish may reduce light transmission and thereby affect SpO2accuracy.
The pulse oximetry device integrated in SOMNOtouch™ RESP is calibrated to
determine the percentage of arterial oxygen saturation of functional haemoglobin.
Significant levels of dysfunctional haemoglobin such as carboxyhemoglobin or
methemoglobin may affect the accuracy of the measurement.
Cardiogreen and other intravascular dyes, depending on the concentration, may
affect the accuracy of the SpO2measurement.
Check all cables and connections for damage before using this device.
Damaged parts must be replaced immediately. Please contact SOMNOmedics or
your SOMNOmedics Distributor.
If the device is damaged (e.g. broken case) it has to be taken out of service.
Misuse of the pulse oximeter sensor with an increased pressure over a prolonged
period can lead to a barotrauma.
For patients with pacemakers we recommend placing the SOMNOtouch™ RESP on
the abdominal region rather than the thoracic region. We recommend placing the
device at least 7 inches away from the pacemaker. Following these placement
guidelines, there is no indication for complications in patients with a pacemaker.
Do not use Radio Transmitters and Receivers, High Frequency Devices, CB-Radio
Systems, Cellular Phones, Microwave Ovens, etc. where the electrical field
strength exceeds 3 V/m (in accordance with IEC 60601-1-2) close to
SOMNOtouch™ RESP.
Only sensors designed and supplied by SOMNOmedics may be used with this unit.
All sensors are provided unsterile and should never be sterilized.
This device is not to be used on broken skin. If this device comes in contact with
broken skin/blood, do not reuse this device and discard.
Only accessories recommended by SOMNOmedics are allowed to be connected to
the SOMNOtouch™ RESP.
This device is not designed for usage in Explosive Environmental Conditions.
It is very important to protect the SOMNOtouch™ RESP from temperatures below
0°C and above 50°C. Furthermore the SOMNOtouch™ RESP may not be worn
during swimming and showering.

- 13 -
Operate the device in an environment with the humidity between 15 and 93% non-
condensing.
This device complies with the IP Protection Class “IP22”.
Do not use an autoclave for disinfection and sterilization of the SOMNOtouch™
RESP or any of its accessories, cables and sensors.
Follow the manufactures instructions when using disinfectants. Keep to the
prescribed dose and contact time. Use protective gloves when using aggressive
disinfectant agents.
There was no testing conducted to demonstrate the moisture retention filter for
nasal cannula (SEN123) used is effective in preventing cross-contamination.
Chemicals which are used for cleaning the unit should be stored, prepared and
kept ready in their own marked containers to avoid confusions.
Long-term storage of this device should only be in a closed and dry room to avoid
condensation caused by thermal fluctuations. Do not store the SOMNOtouch™
RESP in places of high temperature exceeding 35° C or under direct sunlight or in
front of a stove. Be sure not to store it under frozen condition. Please also avoid
the areas of high humidity.
Opening the case, repairing or modifying the SOMNOtouch™ RESP in any way will
void the guarantee and might affect the safety of the device. Only SOMNOmedics
and its authorised distributors may repair the unit.
Always use the Docking Station (TOS900) to charge the internal battery.
The device is not to be used while the battery is being charged.
The body strap is made of material commonly used in clothing and non-medical
watchbands, however if redness or swelling of the skin occurs where the band is in
contact, please discontinue use immediately and consult your physician.

- 14 -
4 Installing the DOMINO light Software
Please note the System Requirements for running DOMINO light Software.
Please also note that the Software must be activated by entering a Registration Code.
You will find the file on the installation CD.
Double click on this file to start the installation.
Choose your language and click on button Next>.
Read carfully the information and click the Next button.
Select an installation folder and click the Next button.

- 15 -
Accept the selection “New Installation”by clicking the Next button.
Click “Next”.
Start the installation process by clicking “Next”.
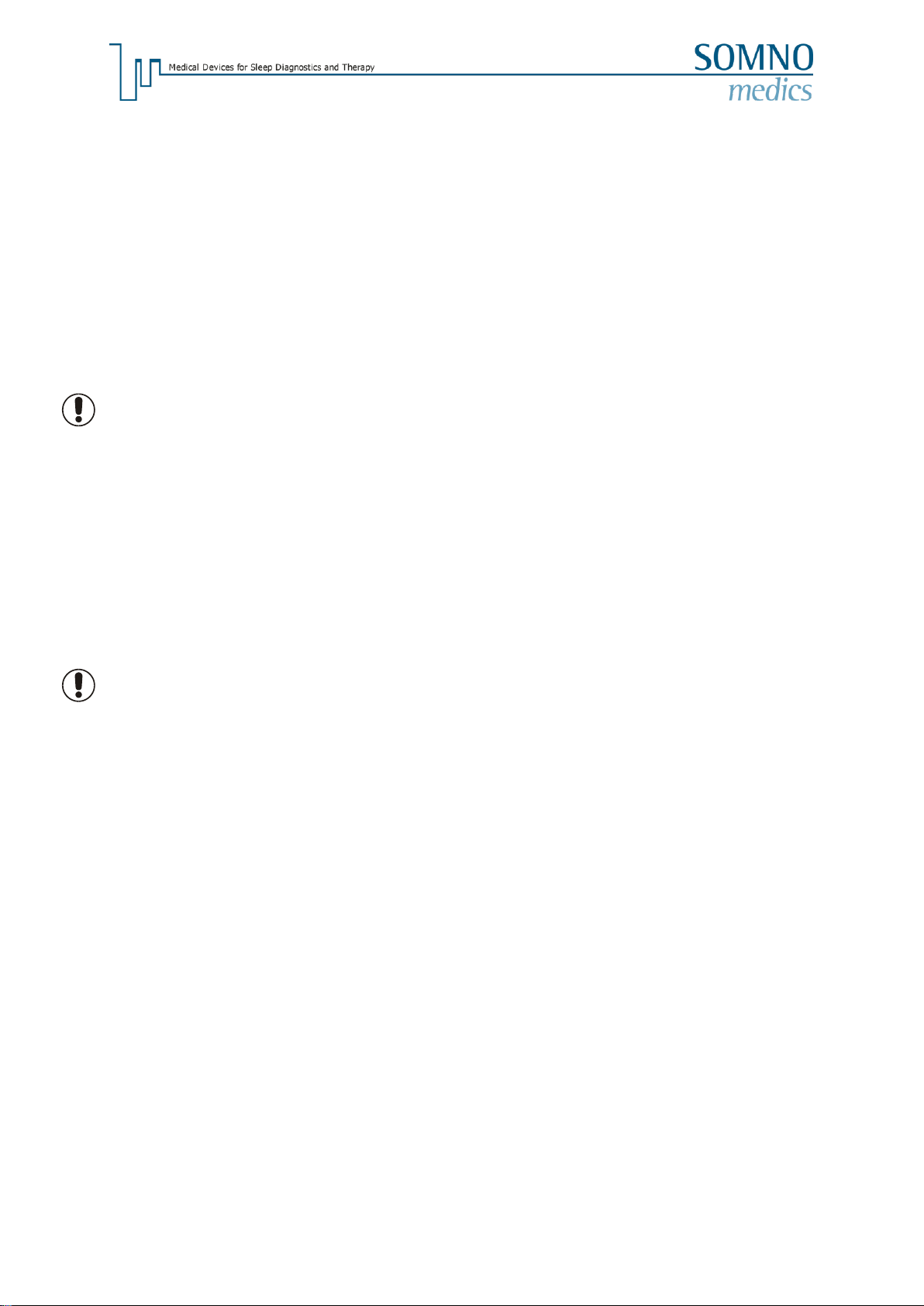
The installation progress will be displayed by a running green bar.
- 16 -
When the installation is complete the software will ask if you want to install the USB driver for the
Docking Station. Leave the default option for the first installation, you can use this to reinstall the USB-
driver later if necessary.
If “Run DOMINO light”is chosen, the software will start immediately after clicking the “Finish”button.
For the driver installation a window of the device installation assistant will open. Confirm each step
with “Next”till the driver installation is finished.
- 17 -
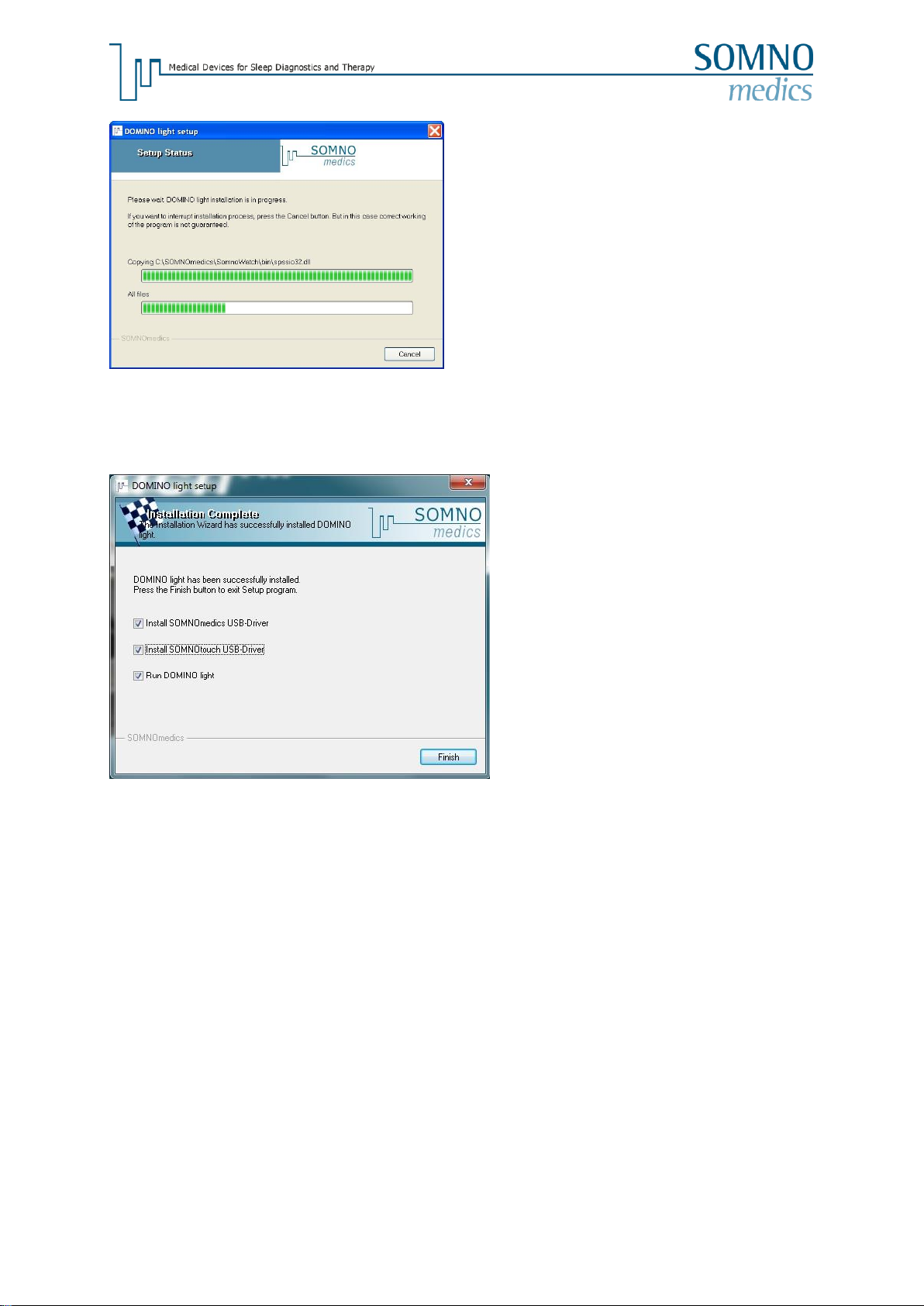
Updating the Software
To update your software version please install the new version in the same directory as the original
version. During the installation you will be asked to choose one of the following installation types.
New analysis, channels and features of the software are updated. The settings of analysis and
analysis templates remain as before.
You can choose which components shall be installed.

- 18 -
All existing settings are overwritten with the new standard settings of the software.
Data safety notes:
Personal patient data is stored on the computer when transferring measurements.
Please take all necessary measures to protect data on the storage, such as i.e.
automatic timed user session log offs, limit physical and network access to the storage
device, use of layered authentication, use strong passwords
Install DOMINO light only from thrustworthy sources such SOMNO-medics GmbH or
your local distributor. Installation of software or firmware from not trustworthy sources
can lead to infection with malware.
It is recommended to use antivirus software and a firewall to protect your PC from
malware and unauthorized access.

- 19 -
5 Manual Docking Station Driver Installation
5.1 Windows 7
1. Connecting the Docking Station
First, connect the Docking Station to a free USB-Slot of your Computer.
After connecting the device with the USB-Port there are two possible options:
Option 1
A dialog window “Update driver software…”appears. In this case continue at #3,
“Automatic driver software installation”.
Option 2
Nothing happens after you have connected the device. In this case move on with #2 “Manual
driver installation”.
2. Manual driver installation
In the Start menu right click „Computer“, then in the context menu left click “Properties”:
The window “System”is opened.
Left click the entry “Device Manager”.

- 20 -
In the window “Device Manager”you will find an entry “Other devices”, underneath this entry
there is a point “SOMNOtouch RESP”:
Right click this entry, then a context menu appears. In this context menu left click on “Update
Driver Software”.
Move on at #3 “Automatic driver installation”.
Other manuals for SOMNOtouch RESP
2
Table of contents
Other SOMNOmedics Blood Pressure Monitor manuals