Technolas Victus User manual

User Manual
VICTUS™ Femtosecond Laser Platform, SW V3.3 SP02
UM-100010770 GB
SKU: 70005780TPV
Version 6
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 1 of 207

Publishing details
User Manual
Version 6
[VICTUS SW V3.3 SP02; PI 125 Kit (REF 90000115) or PI 125 Kit (REF 90000145)]
Manufacturer
Technolas Perfect Vision GmbH
Messerschmittstr. 1 + 3
80992 München
Germany
www.TechnolasPV.com
For Customer Service or Technical Support:
Contact your local Customer Service or Technical Support.
Copyright
This document is protected by copyright. All rights reserved. Notably, no portion of this publication may be reproduced or made
available to third parties, in any form, online or offline, without the written consent of Technolas Perfect Vision GmbH. Further, no
portion of this publication may be translated into a language or code that can be utilized for machines, in particular, data processing
systems, or the internet. This document may exclusively be used to properly use the products described herein in accordance with
the contractual terms for such product.
Copyright © 2017 Technolas Perfect Vision GmbH
Subject to technical changes.
Document no. UM-100010770 GB
Edition: 2017-06-12
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 2 of 207

A General information
A.1 Information about this user manual......................................................................................... A - 1
A.2 Explanation of symbols................................................................................................................... A - 1
A.3 Customer service................................................................................................................................ A - 2
A.4 Product names used in this user manual................................................................................ A - 3
A.5 Reference documents to this user manual............................................................................. A - 3
1 Introduction
1.1 VICTUS™ Femtosecond Laser Platform.................................................................................... 1 - 1
1.2 Scope of delivery................................................................................................................................. 1 - 1
1.3 Use with other medical devices.................................................................................................... 1 - 2
1.4 List of abbreviations.......................................................................................................................... 1 - 2
1.5 Definitions.............................................................................................................................................. 1 - 3
2 Safety
2.1 Intended use.......................................................................................................................................... 2 - 1
2.2 Complications (side effects)........................................................................................................... 2 - 1
2.3 Important safety considerations................................................................................................. 2 - 3
2.4 Responsible organization............................................................................................................... 2 - 3
2.5 Customer responsibilities............................................................................................................... 2 - 4
2.6 Personnel requirements.................................................................................................................. 2 - 4
2.6.1 Role definitions....................................................................................................................................... 2 - 4
2.7 General hazards................................................................................................................................... 2 - 5
2.7.1 Electrical hazard...................................................................................................................................... 2 - 6
2.7.2 Laser radiation hazard.......................................................................................................................... 2 - 7
2.7.3 Fire hazard................................................................................................................................................ 2 - 7
2.7.4 Mechanical hazard................................................................................................................................. 2 - 8
2.7.5 Damage to property............................................................................................................................. 2 - 8
2.8 Warning signals and alarms........................................................................................................... 2 - 9
2.9 Safety devices....................................................................................................................................... 2 - 9
TABLE OF CONTENTS
SKU: 70005780TPV * Version 6 * UM-100010770 GB 1
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 3 of 207

2.10 Labeling................................................................................................................................................ 2 - 11
2.10.1 Labels in the operating room entrance area............................................................................. 2 - 12
2.10.2 Labels on the laser system............................................................................................................... 2 - 13
3 Transport and storage
3.1 Transportation..................................................................................................................................... 3 - 1
3.2 Visual inspection after delivery................................................................................................... 3 - 1
3.3 Storage before installation............................................................................................................ 3 - 2
3.4 Unpacking............................................................................................................................................... 3 - 2
4Installation, commissioning, service, calibration,
decomissioning and disposal
4.1 Installation and comissioning....................................................................................................... 4 - 1
4.1.1 Interface of the patient beds "LS Comfort" and "Patient Support System S60" by
AKRUS GmbH & Co KG......................................................................................................................... 4 - 2
4.2 Service and calibration..................................................................................................................... 4 - 3
4.3 Decomissioning and disposal....................................................................................................... 4 - 3
5 Clinical applications
5.1 Medical indications............................................................................................................................ 5 - 1
5.2 Patient selection criteria.................................................................................................................. 5 - 2
5.2.1 Contraindications................................................................................................................................... 5 - 2
5.3 Available procedures........................................................................................................................ 5 - 3
5.3.1 Cataract procedures.............................................................................................................................. 5 - 3
5.3.2 Corneal procedures............................................................................................................................... 5 - 4
5.3.3 Therapeutic procedures...................................................................................................................... 5 - 4
6 Hardware
6.1 Overview on the components....................................................................................................... 6 - 1
6.2 Operating the hardware.................................................................................................................. 6 - 2
6.2.1 Main unit................................................................................................................................................... 6 - 2
TABLE OF CONTENTS
SKU: 70005780TPV * Version 6 * UM-100010770 GB
2
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 4 of 207

6.2.2 Assistant workstation / surgeon control screen......................................................................... 6 - 3
6.2.2.1 Adjusting the monitor settings......................................................................................................... 6 - 3
6.2.3 Start-up panel.......................................................................................................................................... 6 - 4
6.2.3.1 Switching the laser system on and off........................................................................................... 6 - 4
6.2.4 Suction status controller..................................................................................................................... 6 - 5
6.2.4.1 Adjusting the suction status.............................................................................................................. 6 - 5
6.2.5 Force display............................................................................................................................................ 6 - 6
6.2.6 Laser ring light illumination switch................................................................................................. 6 - 6
6.2.6.1 Switching the laser ring light illumination on and off.............................................................. 6 - 7
6.2.7 Camera focus switch............................................................................................................................. 6 - 7
6.2.7.1 Adjusting the camera focus............................................................................................................... 6 - 7
6.2.8 Spacer cone.............................................................................................................................................. 6 - 7
6.2.8.1 Protecting the spacer cone................................................................................................................ 6 - 8
6.2.9 Surgical microscope and its illumination...................................................................................... 6 - 8
6.2.9.1 Adjusting the surgical microscope illumination...................................................................... 6 - 10
6.2.10 Footswitches......................................................................................................................................... 6 - 11
6.2.11 Patient bed............................................................................................................................................. 6 - 12
6.2.11.1 Referencing the patient bed........................................................................................................... 6 - 12
6.3 Cleaning instructions..................................................................................................................... 6 - 13
7 Accessories and tools
7.1 Accessories............................................................................................................................................. 7 - 1
7.2 Tools.......................................................................................................................................................... 7 - 2
8 Software
8.1 Working with the software............................................................................................................. 8 - 1
8.1.1 Starting and closing the software.................................................................................................... 8 - 1
8.1.2 Elements of the ‘Main menu’ screen............................................................................................. 8 - 5
8.1.3 Elements of the treatment screens.................................................................................................. 8 - 6
8.1.3.1 Treatment-specific elements........................................................................................................... 8 - 11
8.1.4 Checking and modifying common settings.............................................................................. 8 - 17
8.1.5 Checking treatment licenses........................................................................................................... 8 - 18
8.1.6 Restarting the software after an emergency shutdown....................................................... 8 - 18
8.2 User administration........................................................................................................................ 8 - 19
TABLE OF CONTENTS
SKU: 70005780TPV * Version 6 * UM-100010770 GB 3
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 5 of 207

8.2.1 User profiles and specific rights..................................................................................................... 8 - 19
8.2.2 Adding users......................................................................................................................................... 8 - 20
8.2.3 Editing registered user accounts................................................................................................... 8 - 21
8.3 System test description................................................................................................................. 8 - 21
8.4 Patient / treatment administration......................................................................................... 8 - 23
8.4.1 Adding patients................................................................................................................................... 8 - 24
8.4.2 Selecting patients................................................................................................................................ 8 - 25
8.4.3 Editing patient data............................................................................................................................ 8 - 26
8.4.4 Deleting patient data......................................................................................................................... 8 - 26
8.4.5 Selecting treatments for execution.............................................................................................. 8 - 27
8.4.6 Creating treatments........................................................................................................................... 8 - 28
8.4.6.1 Creating treatments based on default values........................................................................... 8 - 30
8.4.6.2 Creating treatments based on a template................................................................................. 8 - 31
8.4.7 Editing treatments.............................................................................................................................. 8 - 32
8.4.8 Deleting treatments........................................................................................................................... 8 - 32
8.4.9 Deleting treatment templates........................................................................................................ 8 - 33
8.4.10 Treatment records............................................................................................................................... 8 - 33
8.5 Procedure parameters................................................................................................................... 8 - 34
8.5.1 Parameters for capsulotomy........................................................................................................... 8 - 36
8.5.2 Parameters for lens fragmentation............................................................................................... 8 - 37
8.5.3 Parameters for arcuate incisions.................................................................................................... 8 - 40
8.5.4 Parameters for corneal incisions.................................................................................................... 8 - 43
8.5.4.1 Schematic overview on corneal incisions................................................................................... 8 - 46
8.5.5 Parameters for flap.............................................................................................................................. 8 - 48
8.5.6 Parameters for penetrating keratoplasty.................................................................................... 8 - 50
8.5.7 Parameters for lamellar keratoplasty........................................................................................... 8 - 51
8.5.8 Parameters for crosslinking............................................................................................................. 8 - 53
8.5.9 Parameters for ICRS tunnel.............................................................................................................. 8 - 54
8.5.9.1 Schematic overview on ICRS tunnel incisions........................................................................... 8 - 59
9 Treatment
9.1 Preparing the laser system for a treatment day.................................................................. 9 - 1
9.2 Maintaining the laser system after a treatment day......................................................... 9 - 1
9.3 Performing the daily system test................................................................................................ 9 - 2
TABLE OF CONTENTS
SKU: 70005780TPV * Version 6 * UM-100010770 GB
4
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 6 of 207

9.3.1 Chiller submenu..................................................................................................................................... 9 - 2
9.3.2 Performing the laser position test................................................................................................... 9 - 3
9.3.3 Performing the vacuum test.............................................................................................................. 9 - 4
9.3.4 Performing the pressure test............................................................................................................. 9 - 5
9.3.5 Performing the power test................................................................................................................. 9 - 6
9.3.6 Performing the depth test.................................................................................................................. 9 - 7
9.4 Daily routine.......................................................................................................................................... 9 - 8
9.4.1 When performing a treatment.......................................................................................................... 9 - 8
9.4.2 Positioning the patient on the patient bed.................................................................................. 9 - 9
9.4.2.1 Using a headrest with integrated joystick.................................................................................. 9 - 10
9.4.2.2 Using a headrest with external joysticks..................................................................................... 9 - 11
9.4.3 Connecting the PI 125 to the spacer cone and positioning the suction clip................. 9 - 11
9.4.3.1 Connecting the PI 125 (REF 90000115) to the spacer cone and positioning the suction
clip (REF 90000115)............................................................................................................................. 9 - 13
9.4.3.2 Connecting the PI 125 (REF 90000145) to the spacer cone and positioning the suction
clip (REF 90000145)............................................................................................................................. 9 - 18
9.4.4 Docking the patient to the spacer cone...................................................................................... 9 - 22
9.4.4.1 For capsulotomy and lens fragmentation procedures using a headrest with inte‐
grated joystick...................................................................................................................................... 9 - 23
9.4.4.2 For capsulotomy and lens fragmentation procedures using a headrest with external
joysticks................................................................................................................................................... 9 - 25
9.4.4.3 For all other procedures using a headrest with integrated joystick................................. 9 - 26
9.4.4.4 For all other procedures using a headrest with external joysticks.................................... 9 - 27
9.4.5 Repositioning the suction clip........................................................................................................ 9 - 28
9.4.6 Completing a treatment................................................................................................................... 9 - 29
9.5 Performing a cataract treatment.............................................................................................. 9 - 30
9.5.1 Preparing a cataract treatment...................................................................................................... 9 - 30
9.5.2 Performing a cataract treatment supported by a 2S-OCT.................................................... 9 - 31
9.5.3 Performing a cataract treatment supported by a TD-OCT................................................... 9 - 44
9.6 Performing a corneal treatment............................................................................................... 9 - 51
9.6.1 Performing a flap procedure........................................................................................................... 9 - 51
9.7 Performing a therapeutic treatment...................................................................................... 9 - 55
10 Troubleshooting chart
11 Technical data
TABLE OF CONTENTS
SKU: 70005780TPV * Version 6 * UM-100010770 GB 5
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 7 of 207

11.1 General specifications.................................................................................................................... 11 - 1
11.2 Operating conditions..................................................................................................................... 11 - 3
11.3 Laser specifications......................................................................................................................... 11 - 4
11.4 Transport and storage conditions............................................................................................ 11 - 4
11.5 Electrical connection....................................................................................................................... 11 - 4
12 Index
13 Appendix
TABLE OF CONTENTS
SKU: 70005780TPV * Version 6 * UM-100010770 GB
6
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 8 of 207

A General information
To order additional copies of this user manual:
If additional copies of this user manual are required, refer to the SKU number on the
cover page and contact your Authorized Technolas Perfect Vision Representative.
A.1 Information about this user manual
This user manual enables safe and efficient handling of the product, its accesso‐
ries and tools. It must be kept close to the product where it is permanently acces‐
sible to the personnel.
Before starting any treatments, the personnel must read the manual thoroughly
and understand its contents. Users must comply with all specified safety instruc‐
tions and operating instructions to ensure safe operation.
In addition, local accident prevention regulations and general safety instructions
must be observed in the area where the product is used.
The product images in this user manual are intended to provide a basic under‐
standing of the product and may differ from the actual design you have on site.
Screenshots were created using an exemplary patient and exemplary values.
Depending on your system’s configuration the depicted screenshots may not be
identical to the screen shown on your system.
The software user interface may only be available in English. Where applicable,
the Appendix section of the user manual contains a list of the English software
texts together with the appropriate translation.
A.2 Explanation of symbols
Safety notes in this user manual are introduced with symbols and a signal word
that describe the severity of the hazard.
Follow these safety notes and proceed with caution to avoid accidents, personal
injury, damage to property, and to ensure patient safety.
This symbol and the word "DANGER" indicate an imminent hazardous situa‐
tion that, if not avoided, could cause death or serious injury.
This symbol and the word "WARNING" indicate a possible hazardous situa‐
tion that, if not avoided, could cause death or serious injury.
This symbol and the word "CAUTION" indicate a possible hazardous situa‐
tion that, if not avoided, could result in minor or moderate injury.
This symbol and the word "NOTICE" indicate a possible hazardous situation
that, if not avoided, could result in damage to property or the environment.
Safety notes
LDANGER
LWARNING
LCAUTION
NOTICE
GENERAL INFORMATION A
SKU: 70005780TPV * Version 6 * UM-100010770 GB A - 1
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 9 of 207
This symbol emphasizes useful tips and recommendations as well as information for
the efficient and trouble-free use of the product.
This field shows examples of previous instructions or information.Example of ...
Safety notes are included in step-by-step instructions. This format allows the user
to be aware of safety issues while performing tasks. The safety symbols are also
part of the step-by-step instructions.
The following symbols and markings are used in this manual to indicate guide‐
lines, descriptions of results, lists, and references:
Symbol Indicates
Step-by-step instructions
ðCondition or automatic sequence as a result of
action
References to chapters in this user manual
Lists or list entries without a certain sequence
[Ctrl] Keys on the keyboard, mouse buttons, key switches,
hardware switches, or hardware buttons
‘Start’ Active (clickable) GUI elements
‘Patient selection’ Passive elements
c:\Program\.. Text input
‘Administration
èUser Registry’
Menu path
A.3 Customer service
Our customer service division is available to provide technical support and infor‐
mation. If any questions arise that are not answered in this user manual, do not
hesitate to contact your Authorized Technolas Perfect Vision Representative for
assistance.
Hints, recommendations,
and examples
Safety notes in step-by-step
instructions
Symbols in this user manual
GENERAL INFORMATION
A
SKU: 70005780TPV * Version 6 * UM-100010770 GB
A - 2
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 10 of 207
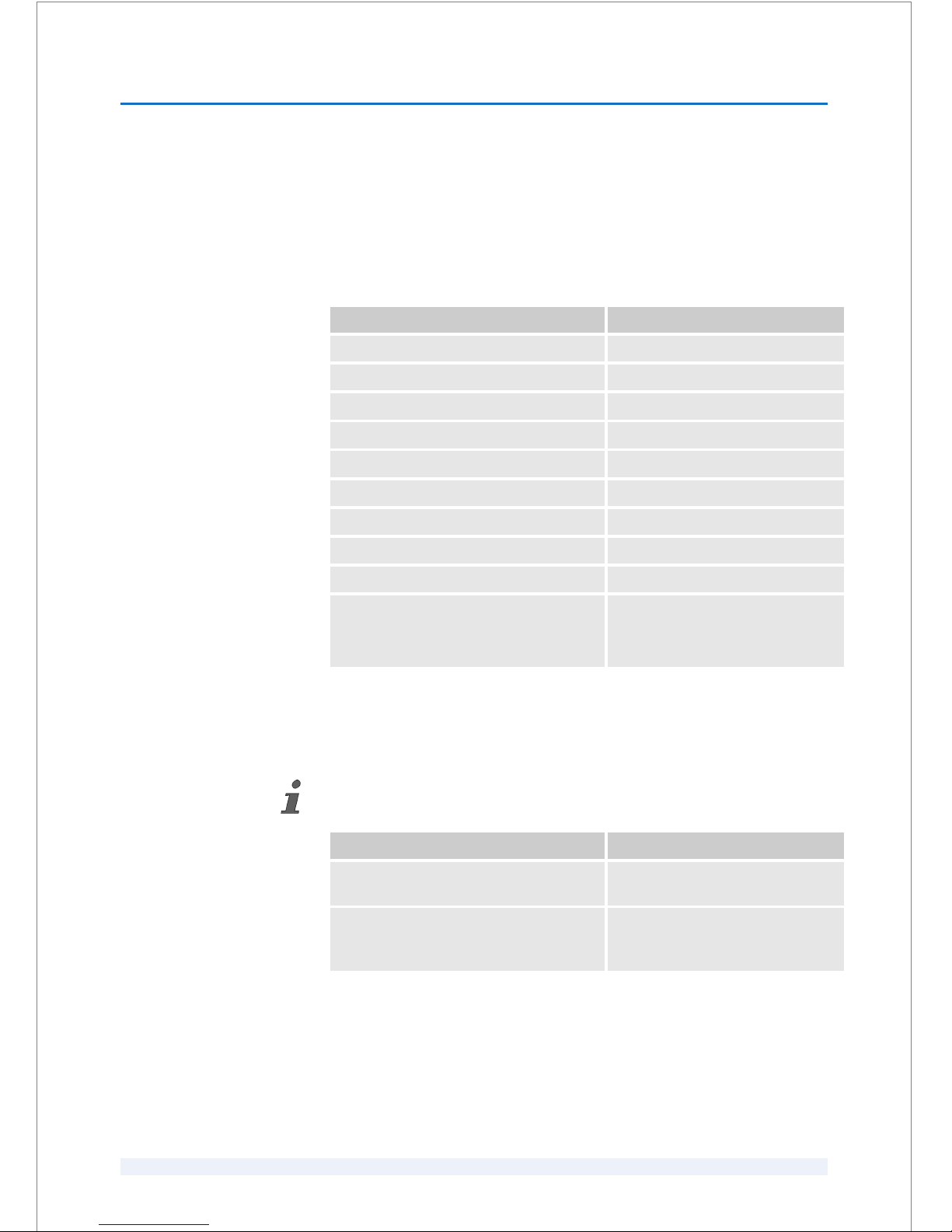
A.4 Product names used in this user manual
For the sake of better readability and depending on the context, product names
may appear in abbreviated form.
Complete product name Name used in this user manual
Patient Interface 125 Kit in general PI 125 Kit
Patient Interface 125 Kit (REF 90000115) PI 125 Kit (REF 90000115)
Patient Interface 125 Kit (REF 90000145) PI 125 Kit (REF 90000145)
Patient Interface 125 in general PI 125
Patient Interface 125 (REF 90000115) PI 125 (REF 90000115)
Patient Interface 125 (REF 90000145) PI 125 (REF 90000145)
Suction Clip 125 in general suction clip
Suction Clip 125 (REF 90000115) suction clip (REF 90000115)
Suction Clip 125 (REF 90000145) suction clip (REF 90000145)
VICTUSäFemtosecond Laser Platform VICTUSäFemtosecond Laser
Platform
or generic term 'laser system'
A.5 Reference documents to this user manual
The following reference documents are related to this user manual.
Document number Document title
GFO-100012286 Product configuration for VICTUS™
Femtosecond Laser Platform
ADD-100011422 Addendum to the user manual
"VICTUSäFemtosecond Laser Plat‐
form, SW V3.3 SP02" (English)
GENERAL INFORMATION A
SKU: 70005780TPV * Version 6 * UM-100010770 GB A - 3
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 11 of 207

GENERAL INFORMATION
A
SKU: 70005780TPV * Version 6 * UM-100010770 GB
A - 4
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 12 of 207

1 Introduction
1.1 VICTUS™ Femtosecond Laser Platform
The VICTUS™ Femtosecond Laser Platform is an ophthalmologic system based on
femtosecond technology and is used to treat a wide variety of ophthalmologic
indications.
The following treatments are available:
The laser system is able to perform steps of a cataract treatment.
For this purpose, an additional OCT (optical coherence tomography) device is
available to help plan the procedure and monitor the treatment.
For details refer to
Ä
Chapter 5.3.1 ‘Cataract procedures’ on page 5 - 3.
The laser system allows very precise, preprogrammed, 3-dimensional patterns to
be cut into the cornea of the human eye. These patterns are cut using infrared
light pulses with spot sizes of approximately 10 μm and pulse duration of several
hundred femtoseconds. The infrared light is focused into the cornea and induces
optical breakdown or photodisruption with practically no heat. This results in the
formation of gas bubbles.
The user can arrange these patterns in various ways, so that various surgical inci‐
sions are possible, for example, a laser-generated LASIK flap.
For details refer to
Ä
Chapter 5.3.2 ‘Corneal procedures’ on page 5 - 4.
Furthermore, the laser system is able to perform various therapeutic treatments.
For details refer to
Ä
Chapter 5.3.3 ‘Therapeutic procedures’ on page 5 - 4.
1.2 Scope of delivery
nVICTUS™ Femtosecond Laser Platform
nUser manual "VICTUS™ Femtosecond Laser Platform, SW V3.3 SP02"
nAddendum to the user manual "VICTUS™ Femtosecond Laser Platform,
SW V3.3 SP02" (English)
nPatient bed (delivered with 2 fuses)
n–User manual "Laser Patient Bed LS Comfort" by AKRUS GmbH & Co KG or
– User manual "Patient Support System PSS S60" by AKRUS GmbH & Co KG
nAccessories and tools (refer to
Ä
Chapter 7 ‘Accessories and tools’ on page 7 - 1)
– Plug adapter
Cataract treatments
Corneal treatments
Therapeutic treatments
INTRODUCTION 1
SKU: 70005780TPV * Version 6 * UM-100010770 GB 1 - 1
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 13 of 207

Connect only items that have been specified as part of the
VICTUS™ Femtosecond Laser Platform ME system or specified as being com‐
patible with the VICTUS™ Femtosecond Laser Platform ME system!
The following items form the VICTUS™ Femtosecond Laser Platform ME
system:
– VICTUS™ Femtosecond Laser Platform
–Patient bed with swivel position 16,5°, 60° or 70°
– Uninterruptible power supply (UPS): see relevant customer configura‐
tion sheet (transient currents up to 16 A possible)
The following parts of the VICTUS™ Femtosecond Laser Platform ME system are
suitable for use within the patient environment:
nVICTUS™ Femtosecond Laser Platform
nPatient bed with swivel position 16,5°, 60° or 70°
1.3 Use with other medical devices
Do not use VICTUS™ Femtosecond Laser Platform together with medical
devices other than the devices described in this section.
Patient beds "LS Comfort" and "Patient Support System S60" by AKRUS
GmbH & Co KG
These patient beds are medical devices that are approved in accordance with the
Medical Device Directive. Technolas Perfect Vision GmbH has assessed their use in
combination with VICTUS™ Femtosecond Laser Platform. Technolas Perfect Vision
GmbH allows only specific versions of the "LS Comfort" or the "Patient Support
System S60" patient bed to be used with VICTUS™ Femtosecond Laser Platform. It
must be installed by an Authorized Service Technician. For information about
how to operate the patient bed refer to the user manual "Laser Patient Bed LS
Comfort" or "Patient Support System PSS S60" by AKRUS GmbH & Co KG. Refer to
Ä
Chapter 4.1.1 ‘Interface of the patient beds "LS Comfort" and "Patient Support
System S60" by AKRUS GmbH & Co KG’ on page 4 - 2 for the interface description.
1.4 List of abbreviations
ACD Anterior Chamber Depth
AI Arcuate Incisions
BSS Balanced Salt Solution
CXL Crosslinking
D Diopter
EHD Exit Height Difference
EMC Electromagnetic Compatibility
ME system
LWARNING
LWARNING
INTRODUCTION
1
SKU: 70005780TPV * Version 6 * UM-100010770 GB
1 - 2
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 14 of 207
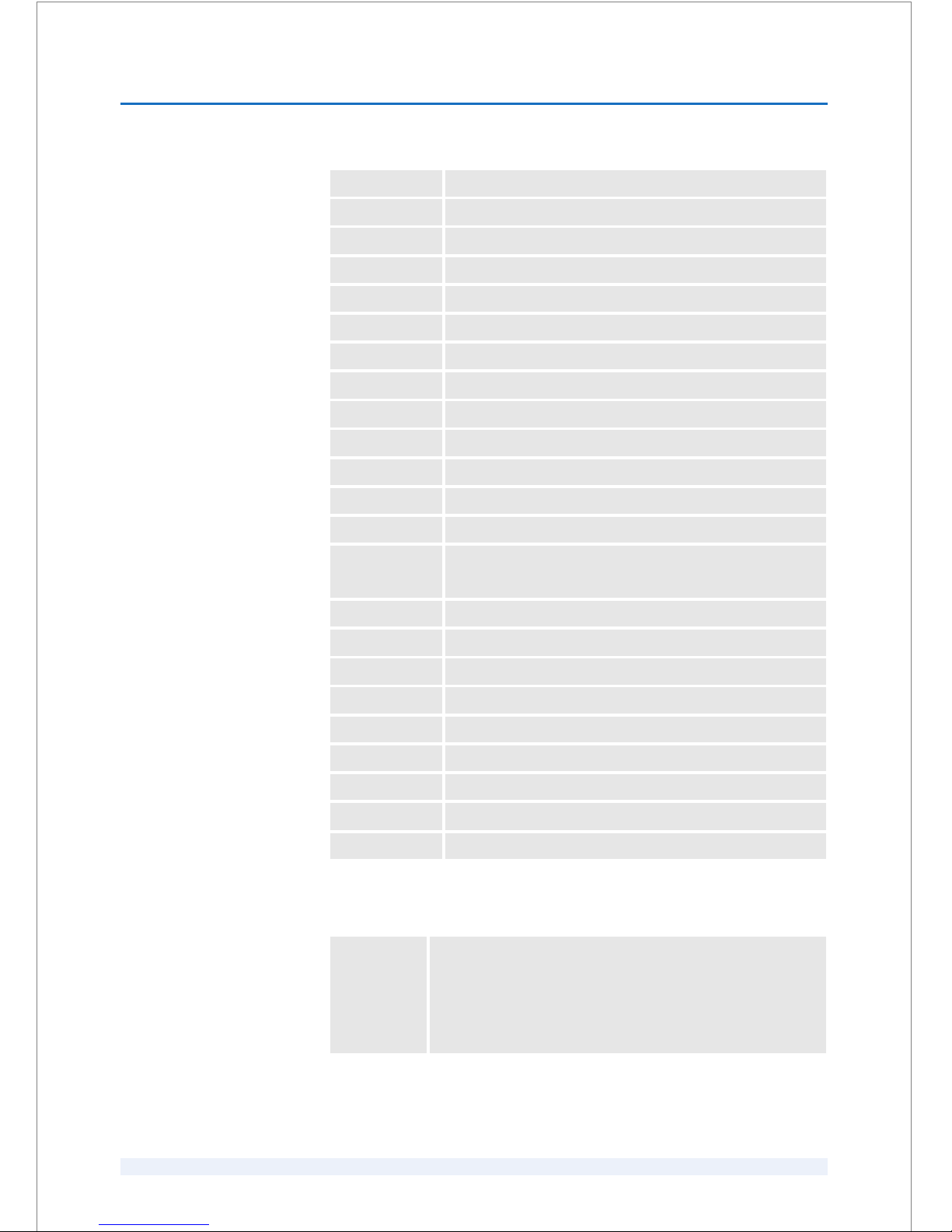
fs femtosecond
GUI Graphical User Interface
H0 Height Zero
hPa hecto Pascal
ICRS Intracorneal Ring Segments
LASER Light Amplification by Stimulated Emission of Radiation
LASIK Laser-Assisted In Situ Keratomileusis
LCD Liquid Crystal Display
LED Light-Emitting Diode
LKP Lamellar Keratoplasty
LPS Laser Position Sensor
LRCS Laser Refractive Cataract Surgery
LVC Laser Vision Correction
ME Medical Electronic
e.g. Medical Electronic Equipment
NOHD Nominal Ocular Hazard Distance
OCT Optical Coherence Tomography
PETG Polyethylene Terephthalate Glycol
PI Patient Interface
PIK Patient Interface Kit
PKP Penetrating Keratoplasty
UPS Uninterruptible Power Supply
®Registered trade mark
äTrade mark
1.5 Definitions
LRCS LRCS indicates a set of procedures that includes the following:
ncapsulotomy
nlens fragmentation
narcuate incisions
ncorneal incisions
INTRODUCTION 1
SKU: 70005780TPV * Version 6 * UM-100010770 GB 1 - 3
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 15 of 207

INTRODUCTION
1
SKU: 70005780TPV * Version 6 * UM-100010770 GB
1 - 4
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 16 of 207
2 Safety
Please ensure that only the corresponding user manual with the correct software ver‐
sion (refer to the label "Installed software" in
Ä
Chapter 2.10.2 ‘Labels on the laser
system’ on page 2 - 13) is used as a reference material!
2.1 Intended use
The VICTUS™ Femtosecond Laser Platform has been designed and constructed
exclusively for the intended use described in this section.
The VICTUS™ Femtosecond Laser Platform is a femtosecond laser that is used to
perform cataract and refractive surgery on human eyes (
Ä
Chapter 5.1 ‘Medical
indications’ on page 5 - 1). Patients may be treated provided they do not exhibit
any condition that may pose a contraindication as discussed in
Ä
Chapter 5.2.1
‘Contraindications’ on page 5 - 2.
The laser system must only be used in dry, dust-free rooms that comply with the
cleanliness standards for operating rooms.
The laser system must only be used with the installed software.
The laser system is also intended to be used only as described in the instructions
in this user manual.
Any use that exceeds, or is different from, the specified intended use is consid‐
ered to be misuse.
Danger of injury due to misuse!
Misuse of the laser system may lead to dangerous situations and severe inju‐
ries.
– Do not use the laser system on patients who come under the exclusion
criteria (
Ä
Chapter 5.2.1 ‘Contraindications’ on page 5 - 2).
– Do not use the laser system with devices, accessories and tools that are
not specified in this user manual.
– Do not use the laser system with software that is not authorized and cer‐
tified by Technolas Perfect Vision GmbH.
– Connect only items that have been specified as part of the
VICTUS™ Femtosecond Laser Platform ME system or specified as being
compatible with the VICTUS™ Femtosecond Laser Platform ME system.
Claims of any type due to damage arising from misuse are excluded.
2.2 Complications (side effects)
Potential general complications resulting from VICTUS procedures include,
but are not limited to the following:
LWARNING
SAFETY 2
SKU: 70005780TPV * Version 6 * UM-100010770 GB 2 - 1
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 17 of 207

nCorneal abrasion or defect
nPain
nBleeding
nInflammation
nElevated intraocular pressure
nIncomplete procedure resulting from treatment interruption caused for
example by suction loss or insufficient contact pressure
nHemorrhage
nDecentration of planned procedure
Potential complications resulting from anterior capsulotomy or lens frag‐
mentation include, but are not limited to the following:
nIncomplete capsulotomy or lens fragmentation
In this case, the surgeon may elect to complete the procedure using tradi‐
tional surgery methods.
nCapsular tear and/or rupture
nDamage to intraocular structures
nMiosis
Potential complications resulting from creation of corneal flaps or corneal
cuts / incisions include, but are not limited to the following:
nCorneal edema
nEpithelial ingrowth
nIncomplete flap or corneal cuts / incisions creation
nFlaps and LKP only:
–Flap striae
– Flap / graft tearing or incomplete lift
– Photophobia
– Thick or thin flap / graft
– Vertical gas breakthrough
– Anterior chamber gas bubbles
– Opaque bubble layer
nLKP only:
– Corneal perforation
ICRS tunnel incisions only:
nAnterior or posterior corneal penetration
Potential complications are not limited to those included in this list. These complica‐
tions may be surgically or medically managed using currently accepted techniques.
SAFETY
2
SKU: 70005780TPV * Version 6 * UM-100010770 GB
2 - 2
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 18 of 207
2.3 Important safety considerations
Danger of injury due to user negligence!
Always double-check the following before treatment:
– Are you treating the right patient?
–Did you select the correct treatment?
– Was the correct eye selected?
– Does the entered data match the patient to be treated?
Failure to verify any of the above-mentioned aspects may result in serious
permanent patient injury.
Danger of injury for persons with cardiac pacemakers!
Negative influence on the pacemaker, such as electromagnetic interference,
could occur during laser operation.
– During laser operation, no person— medical personnel or patients—
with a cardiac pacemaker should be present in the treatment room.
Property damage due to improper stacking or locating of non-intended
devices next to the product!
The product is very sensitive. Improper stacking or locating of non-intended
devices next to the product may lead to equipment damage or influence
concerning EMC. If adjacent or stacked use is necessary, the product should
be observed to verify normal operation in the configuration in which it will
be used.
– Do not stack or locate any non-intended devices next to the product
during operation.
The medical electrical equipment implies special precautions regarding EMC and
needs to be installed and put into service according to the EMC information provided
in
Ä
Appendix A ‘EMC declaration’ on page 13 - 3.
The medical electrical equipment may be affected by portable and mobile radio fre‐
quency communication equipment.
2.4 Responsible organization
The responsible organization can be, for example, a public hospital, a private
person (ophtalmologist), or a private clinic.
Education and training is included in the term 'use'.
nThe responsible organization must ensure that hazards of network coupling
of the device must be observed. Such hazards include, for example, virus pro‐
tection, data security, network protection by firewall systems, and ensuring
sufficient bandwidth.
nSystem modifications and use in combination with other equipment is
limited to TPV-approved equipment. Otherwise, the responsible organization
is in charge of proper risk management.
LWARNING
LWARNING
LWARNING
SAFETY 2
SKU: 70005780TPV * Version 6 * UM-100010770 GB 2 - 3
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 19 of 207

2.5 Customer responsibilities
The laser system is used for commercial purposes. Laser system users are there‐
fore required to observe their obligations with regard to occupational health and
safety.
In addition to the safety instructions provided in this manual, the safety, accident
prevention, and environmental regulations applicable at the place of installation
must be observed, particularly as follows:
nThe customer must ensure that personnel who handle the laser system read
and understand the manual.
nThe customer must ensure that unauthorized persons do not have access to
the operating room and the laser system.
nThe customer must ensure that the service and calibration intervals specified
in this manual are observed.
nThe customer must provide all users with personal protective equipment, if
necessary.
nThe customer must ensure that all warning signs are prominently displayed
on the access to the operating room.
nThe customer must ensure that the entrance to the operating room is
equipped with a light that shows if the operating room is in use.
2.6 Personnel requirements
Danger of injury if persons are unauthorized or insufficiently qualified!
If unauthorized or unqualified persons perform work on the laser system or
are in the danger zone of the laser system, hazards may arise that can cause
serious injury and substantial damage to property.
– All treatments must be performed by appropriately qualified persons.
–Unauthorized persons must not use the product.
All persons are expected to perform their work in a reliable manner. Persons with
impaired reactions due to the consumption of drugs, alcohol, medication, or any
other reason are prohibited.
2.6.1 Role definitions
Assistant
Assistants are trained for the special area of responsibility in which they are
involved. Assistants know the content of all regulations, guidelines, and standards
which are applicable for the safe use of the system and can implement the
requirements mentioned in them.
Assistants have been trained by Technolas Perfect Vision GmbH staff or personnel
authorized by Technolas Perfect Vision GmbH in proper handling of the system.
Assistants can safely perform the work assigned to them and can recognize, eval‐
uate, and avoid potential hazards to themselves or the patient because of their
professional medical training and experience.
LWARNING
Personnel in general
SAFETY
2
SKU: 70005780TPV * Version 6 * UM-100010770 GB
2 - 4
TPV Controlled Document / Status: Effective / Effective Date: Jun 14, 2017 2:38:46 PM
UM-100010770 / Version 6 / Page 20 of 207
Table of contents
Popular Medical Equipment manuals by other brands

Microlife
Microlife MedGem Frequently asked questions

Otto Bock
Otto Bock 757M20 Series Instructions for use

NRS Healthcare
NRS Healthcare L22056 User instructions

RWD
RWD R640-S1 user manual

Direct Supply
Direct Supply Panacea Hot and Cold Packs owner's manual

Boston Scientific
Boston Scientific Precision Montage Information for Prescribers