Tremitas Tremipen Home User manual

Instructions for Use
Tremipen® Home
for the
1304
EN


We, the Tremitas GmbH, declare in sole responsibility
as the Legal Manufacturer, that the medical device
Tremipen® Home
is in compliance with the Directive MDD 93/42/EEC
and the Austrian medical-device-law BGBI. 657/1996.
The CE-label on the device documents
the accordance to this.
The full text about the Declaration of Conformity is
available on our website: www.tremitas.com
Declaration of Conformity
1304

1. Table of Contents
1. Table of Contents 2
2. General Information 4
2.1 General Information about the Product 4
2.1.1 Name – Tradename 4
2.1.2 Reference to Variants 4
2.1.3 Reference to allowed Accessories 4
2.1.4 Safekeeping of the IfU 4
2.1.5 Scope of Delivery | Parts of the Device 4
2.2 Legal Manufacturer of the Tremipen® 5
2.3 Issue Date of the IfU 5
3. Intended Use | Purpose 5
4. How to use the Tremipen® safely 6
4.1 Handling of the Tremipen®
– How to make Measurements 6
4.1.1 Measurement Positions (Examples) 7
4.1.3 Symbols on the Tremipen® Display 10
4.1.4 Tremor Amplitude (Power of Main Peak) 11
4.1.5 Medical Decisions based on Tremipen® 14
4.2 User (Training, Knowledge) 15
4.3 Medical Indications 15
4.4 Where, how long and how often should
the Tremipen® be used? 15
4.5 Contraindications 15
4.6 Exclaimers 16
4.7 Application Environment 16
4.7.1 Temperature 16
4.7.2 Humidity 16
4.7.3 Brightness 16
4.7.4 Electromagnetic Compatibility | Radiation 16
4.7.5 Pressure 17
4.7.6 Heights above the Sea Level 17
4.8 Storage | Safekeeping | Transport 17
4.9 Time until Retesting 17
4.10 Installation | Battery Usage |
Battery Changing 18
4.10.1 Installation 18
4.10.2 Battery Usage and Changing 19
4.10.3 Typical Battery Lifespan 19
4.10.4 Controlling the Battery Status 19
4.11 Connections with and to
Main Power Supplies 19

4.12 Combination | Compatibility
with other Products and Devices 20
4.13 Distance to RF
Communications Equipment 20
4.14 Electromagnetic Compatibility (EMC) 20
4.14.1 Electromagnetic Classication
of the Tremipen® 21
4.14.2 Summary of Electromagnetic Emissions 21
4.14.3 Summary of Electromagnetic Immunity 21
4.14.4 RF Properties 22
4.15 Accessories 22
4.16 Service 22
4.17 Repair 22
4.18 Retesting 22
4.19 Inspection | Reevaluation 23
4.20 Cleaning and Disinfection 23
4.21 Decommissioning
Lifecycle of the Tremipen® 24
4.22 Correct Waste Disposal 24
4.22.1 Disposal of Packaging Waste 24
4.22.2 Disposal of the Tremipen® 24
5. Performance and Technical Data 26
5.1 Changes of Technical Data 26
5.2 Rules, Laws and Standards applicable
for the Tremipen® 27
6. Adverse Effects and Residual Risks 27
7. Safety Warnings and Risks 27
8. Denition of Symbols 30
9. Technical Self Diagnosis Function 32
10. Technical Bluetooth® Interface 33
11. Possible Error Causes and Solutions 34
12. Denition of Color Coding and
Identifying Colors 35
13. Special Exclusions 35
14. Information about Safety Reports 35
15. Reference to Clinical Performance 35
16. Allowed Languages for this IfU 35
17. CE-Label 36
18. Warranty and Contact 36
19. Local Tremitas Partner 36

The Tremipen® Home is a class Im medical device used
for tremor quantication. The device can be used for
monitoring purposes and as an assisting tool for
therapeutic processes.
This Instructions for Use (in the following named as
“IfU”) pertains to the Tremipen® Home (in the following
named as Tremipen®), which is delivered to you,
the user. Please read the IfU, in particular the safety
references, very carefully before using the Tremipen®.
Not reading this IfU can lead to the wrong interpretation
of measurement results, wrong measurements and
safety risks. Not following the IfU will lead to a warranty
loss.
2. General Information
2.1 General Information about the Product
2.1.1 Name – Tradename
Tremipen® Home (also Tremipen® Home by Tremitas,
Tremipen®).
2.1.2 Reference to Variants
There are different variants of Tremipen® (e.g.
Tremipen Home®, Tremipen®.
2.1.3 Reference to allowed Accessories
The Tremipen® does not have accessories within the
dened scope of the Medical Device Directive (MDD).
2.1.4 Safekeeping of the IfU
This IfU is based on the standards and rules, which
are valid within the European Union. Please note that
state-specic guidelines and laws abroad could also
apply. Keep the IfU for further use. If you need an
additional IfU, please contact your local Tremipen®
distributor. If you provide the Tremipen® to third parties,
the IfU must also be provided.
2.1.5 Scope of Delivery | Parts of the Device
The scope of delivery includes the following parts:
1 pc. Tremipen®
1 pc. 1.5 V AAA-battery
see point 4.10 in this IfU
1 pc. Tremipen® Instructions for Use
4

Tremipen® is only allowed to be used for the following
purposes:
1. The examination of a physiological
process (uncontrollable shaking of
upper extremities, hands and ngers)
2. The monitoring of diseases (by measuring
the diseases‘ symptoms on a regular basis),
specically (according to ICD 10-GM-2017):
Tremipen® itself has the following device parts:
1. Tremipen® (Case) 4. Grip recess
2. Power-switch | ON Button 5. Battery case
3. Display 6. Battery
3. Intended Use | Purpose
2.2 Legal Manufacturer of the Tremipen®
Tremitas GmbH
Schleppe-Platz 5 | Klagenfurt A-9020 | Austria
www.tremitas.com | oce@tremitas.com
+43 660 55 10 380
2.3 Issue Date of the IfU
Date of last revision: Feb 03rd 2020
Version Number: 1.0
Please contact your local Tremipen® distributor if you
need details about future versions of the IfU.
(6) Battery
(internal)
(1) Tremipen®
(Case)
(2) Power switch |
ON Button
(5) Battery case
(3) Display
(4) Grip recess
5
4. How to use the Tremipen® safely
4.1 Handling of the Tremipen®
– How to make Measurements
•G20.- (Primary Parkinson Syndrome)
•G20.0 (Primary Parkinson Syndrome with missing
or low-level impairment)
•G20.1 (Primary Parkinson Syndrome with moderate
or high-level impairment)
•G25.0 (Essential Tremor)
• G25.2 (Other, not further specied forms of tremor)
3. The monitoring of the symptom tremor
(by measuring the symptom on a regular basis)
Tremitas GmbH does not take any responsibilities
and liabilities for incidents, accidents, defects
and/ or harms and damages, which occur due to a
incorrect or improper use of the Tremipen®!
Tremipen® is NOT a toy – keep away from children!
Tremipen® must only be used as described
in this IfU!
The ON Button of the Tremipen® is a small part,
which could be swallowed. Do not remove it or it can
become a choking hazard.
1. Push the ON Button on the Tremipen®.
2. Take the Tremipen® into your hand and hold it just
like a regular pen; a strong grip is not necessary,
only a loose grip (this is necessary so that the
tremor is not overlapped by a physiological tremor)
— see chapter 4.1.1 for further information.
(Measurements can be made for the right hand, the
left hand or even both with two measurements; this
depends on your dominant side and which side is
more interesting for you and/or your physician for
assessments and evaluations.)
6
For further information and instruction videos please visit
our website www.tremitas.com.
3. Choose one of the standardized positions to
make a tremor measurement. The Tremipen®
provides you a 5 second countdown.
See chapter 4.1.1 for further information.
(These positions can be the rest tremor position,
the postural tremor position, the wing-beating tremor
position etc.).
4. After 5 seconds a beep tone sounds, the progress
bar appears and the measurement begins. For the
next 30 seconds, remain still in the chosen position;
no writing or moving activities are required.
5. After 30 seconds, another beep tone sounds, and
the measurement is over. The display shows the
measurement results; these results are also stored
within the Tremipen®.
6. After 30 seconds, the Tremipen® automatically
turns off and is ready for another measurement.
7. The Tremipen® can be manually shut down by
continuously pressing the ON button for 3 seconds
(this can be done during or after a measurement)
The following rules apply for all measurement positions:
1. The Tremipen® must be held with a loose grip, just
like a regular pen, so that no physiological tremor
can inuence the actual tremor.
2. The thumb and index nger are positioned on
the grip recess, the middle nger supports the
Tremipen® from below.
3. The Tremipen®‘s end with the battery case has to
rest on the back of the hand.
4. The measurement position must be identical for all
measurements if results need to be compared.
5. Throughout the whole measurement process, you
shall not move the hand deliberately
4.1.1 Measurement Positions (Examples)
7
The Tremipen® must only be used with a
closed battery case!

1. Hold the Tremipen® as described above.
2. Place your arms in a relaxed position onto your
upper legs or onto armrests of a chair, as seen in
the picture above.
3. Remain in this position during the whole
measurement process.
1. Hold the Tremipen® as described above.
2. Extend your arms horizontally / forward in a 90°
angle with straightend and lowered shoulders as
seen in the picture above.
Measurement Position for Postural Tremor
(e.g. for Essential Tremor)
Measurement Position for Rest Tremor
(e.g. for Parkinson‘s Disease)
How to hold the Tremipen® during a measurement
8

3. The ngers on the hand, which does not hold the
Tremipen®, should be spread out. Additionally, the
ring nger and the little nger of the hand, which
is holding the Tremipen®, should be spread away
from the palm as far as possible.
4. Remain in this position during the whole
measurement process
1. Hold the Tremipen® as described above.
2. For the wing-beating position, the arms are
positioned just like wings as seen in the picture
above.
3. The hands are held in front of the upper body,
slightly below the chest, the ngertips of both
hands should be close to each other.
4. Remain in this position during the whole
measurement process.
Wing-Beating Position
(e.g. for Essential Tremor)
The Tremipen® determines the following
measurement parameter:
1. The tremor‘s amplitude (intensity of shaking)
– measured in mG (milli-G)
After a measurement, the tremor‘s intensity is shown on
the display in mG.
4.1.2 Interpretation of Measurement Results
9

The following symbol is shown for all measurement
results below 10mG and 3Hz (underow):
The following symbol is shown for all measurement
results over 1750mG (overow):
All measurement results are beyond the measurement
range of the Tremipen® if they are below 10mG and
above 1750mG.
The preperation time was started, the
display shows a countdown (5, 4, 3, 2, 1)
The Tremipen® is in measurement
mode, this is shown with a progress bar
The tremor amplitude is shown; it
consists of a two- to four-digit number,
followed by the unit (mG)
The tremor amplitude was too low
The tremor amplitude was too high
The tremor frquency was too low
The battery is almost depleted / the
battery needs to be changed
The technical self-diagnosis function
was trigggered
The Tremipen® is in Bluetooth®mode
A system error occurred
4.1.3 Symbols on the Tremipen® Display
10

4.1.4 Tremor Amplitude (Power of Main Peak)
The tremor amplitude is a relevant parameter to dene
how strong and intense tremor manifests. The tremor
amplitude was historically measured in milli-Volt or
mV, but the more accurate physical unit for this purpose
is milli-G or mG. 1G equals the acceleration or gravity of
earth and since the accelerometer inside the Tremipen®
measures accelerations, mG is currently used more
often than mV. (Nevertheless, it is possible to convert
the values).
To better understand the relations between the mG unit
and the tremor amplitude, the Tremitas GmbH has used
available clinical data from own clinical trials to create
a comparison between the amplitudes dened in the
“Unied Parkinson’s Disease Rating Scale” (UPDRS)
and the “The Essential Tremor Rating Assessment
Scale” (TETRAS). These scales are only for information
purposes and shall not to be used as exact scales.
(go to page 12 & 13)
11

12

13

The measurement results generated by the Tremipen®
are clinical measurement results that can be be used
within the intended purpose. Nevertheless, the results
and the use of the Tremipen® are NOT meant to be used
for diagnostic or therapeutic purposes by users, who
are not medical experts. This applies for the following,
but not exclusive list of situations:
• Self-diagnosis without a medical expert
• Self-adjustment of medications without a medical
expert
• Self-decided changes in medication without
consulting a medical expert
4.1.5 Medical Decisions based on Tremipen®
14

The Tremipen® is intended to be used by the patients
themselves (lay person), since the intended functioning
requires the patient to hold the object.
Medical experts and professionals can assist during
the preparation or the implementation of the intended
operation.
The Tremipen® itself does not require special training
and knowledge, but the IfU must be understood and
complied with.
The Tremipen® shall only be used for the following
symptoms or diseases (according to ICD-10-GM-2017):
• G20.- (Primary Parkinson Syndrome)
• G20.- (Primary Parkinson Syndrome)
•G20.0 (Primary Parkinson Syndrome with
missing or low-level impairment)
•G20.1 (Primary Parkinson Syndrome with
moderate or high-level impairment)
•G25.0 (Essential Tremor)
• G25.2 (Other, not further specied forms of
tremor)
•Additionally, tremor as a symptom.
•The Tremipen® can be used in clinical
environments, such as hospitals and doctors’
oces, and in home environments.
•The Tremipen® is NOT meant to be used outdoors.
•The measurement process of the Tremipen® takes
approximately 30 seconds.
•Including the activation and other usability
features, the typical duration of use is still below
60 seconds.
•The Tremipen® is intended for multiple
applications.
4.2 User (Training, Knowledge)
4.3 Medical Indications
4.4 Where, how long and how often should
the Tremipen® be used?
Currently, no contraindications are known regarding
the use of the Tremipen®.
4.5 Contraindications
15

The Tremipen® must NOT be used by users,
who are not able – in case of physical and/or
mental impairments – to implement a correct
measurement. In this case, the measurement
must be supervised by qualied personnel or
professionals. Otherwise it could lead to wrong
measurement results.
4.6 Exclaimers
4.7 Application Environment
Exclaimers for incorrect use of the
Tremipen®
Attention: The Tremipen® must only be
used under the following environmental
conditions. Otherwise it could damage
the device or lead to wrong measurement
results.
10°C to 35°C
20% to 80%
4.7.1 Temperature
4.7.2 Humidit y
4.7.3 Brightness
4.7.4 Electromagnetic Compatibility | Radiation
Keep away from heat and direct
sunlight!
See chapter 4.14. Do not use the
Tremipen® in close proximity
to devices, which generate
strong electromagnetic elds
such as magnetic resonance
imaging devices or HF surgical
equipment.
16

Heights above the sea level are bound to
the pressure described in chapter 4.7.5
4.7.5 Pressure
4.7.6 Heights above the Sea Level
700 hPa to 1060 hPa
•If you do not use the Tremipen® for a longer time,
keep it safe within the provided packaging.
•Clean the Tremipen® before storage as described
in chapter 4.20. This is especially important if the
Tremipen® is used by multiple users.
• Protect the Tremipen® against direct sunlight and
other sources of heat and put it into the provided
and specially designed packaging.
• Keep the Tremipen® away from children.
•The Tremipen® must only be transported within
the provided packaging.
•If the Tremipen® is not stored within the
recommended environmental conditions
described within this IfU (heat and coldness), let it
cool down or warm up for at least an hour at room
temperature (approx. 20°C) before using it again.
The Tremipen® is produced for
an overall operating period of
2 years until it has to be retested. This operating period
only be guaranteed if rthe Tremipen® is properly used.
4.8 Storage | Safekeeping | Transport
4.9 Time until Retesting
Protect the Tremipen® against rain,
wetness and humidity to prevent
damages to the device or wrong
measurement results.
17
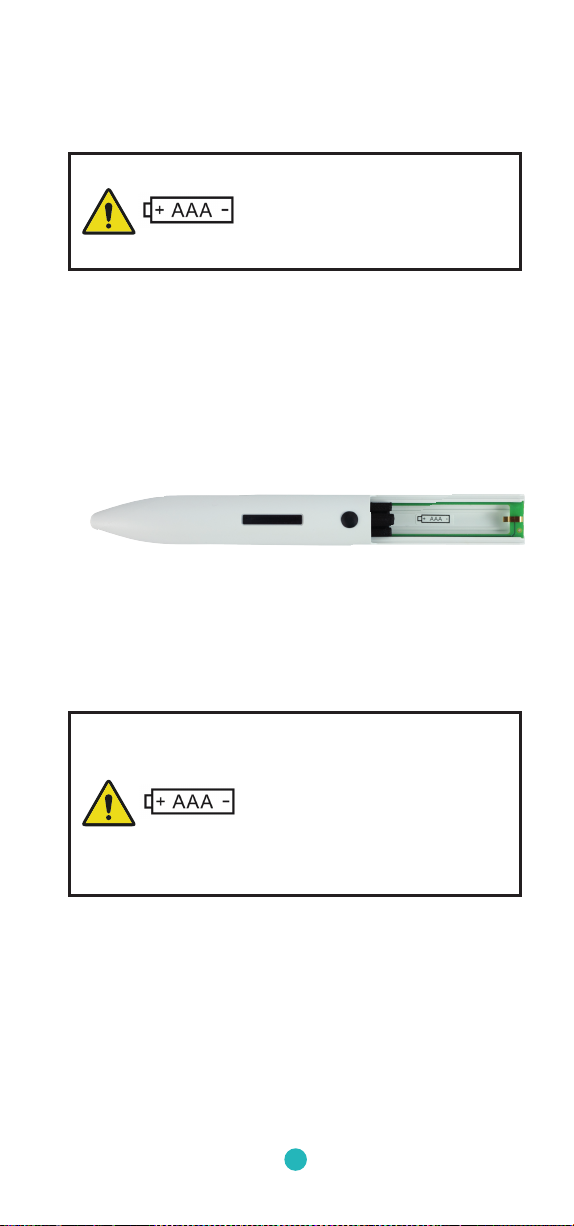
Brand: Varta®
Model: Industrial
Type: AAA Battery (1.5V)
All performance characteristics and functionalities
of the Tremipen® were only tested with this type of
battery! The Tremitas GmbH is not liable for improper
functioning or malfunctions of the Tremipen® due to
the use of other battery types. If alternative batteries
are use d , they have to have at least equ al spe c icatio ns.
1. Take the Tremipen® out of the packaging.
2. Open the battery case.
3. Insert the enclosed battery correctly and carefully;
pay attention to polarity. The positive pole of the
battery must face to the display:
4. Close the battery case. Attention, do not bruise
your ngers!
5. Press the ON Button on the Tremipen® and check if
the device turns on and performs a measurement.
4.10.1 Installation
4.10 Installation | Battery Usage |
Battery Changing
The following steps must be
implemented before the rst
use of the Tremipen®!
For the Tremipen®, only
the following battery or a
battery with at least equal
specications is recommended.
Using a different type could
lead to wrong measurement
results.
18
This manual suits for next models
1
Table of contents
Popular Medical Equipment manuals by other brands
GE
GE Vscan Air manual

Atos Medical
Atos Medical Provox FreeHands FlexiVoice Instructions for use

OPTIKON
OPTIKON PULSAR2 ESP Installation and operating manual

Vermeiren
Vermeiren SLINGS Albatros instruction manual

Integra
Integra MAYFIELD A2101 instruction manual

Dr. Honle
Dr. Honle dermalight 80 operating instructions

Fresenius Kabi
Fresenius Kabi CompoSeal Universal operating instructions

St. Jude Medical
St. Jude Medical Proclaim DRG 3664 Clinician manual

Heraeus Kulzer
Heraeus Kulzer Translux Power Blue Instructions for use

Promeba
Promeba PS-171 user guide
Invacare
Invacare JASMINE user manual

Dräger
Dräger Infinity C700 Quick reference guide