IGEA I-ONE User manual

I-One®
User Manual
Manuale d’uso
Manuel d’utilisation
Handbuch
Manual de usuario
+39 059 699600
+44 01937 547065 +39 059 699600
+49 89 23041765 +39 059 699600


CONTENTS
1. ENGLISH ........................................................................................................................... 2
2. ITALIANO.......................................................................................................................... 22
3. FRANÇAIS ....................................................................................................................... 42
4. DEUTSCH ........................................................................................................................ 63
5. ESPAÑOL......................................................................................................................... 84
1

ENGLISH
IGEA I-ONE - User Manual
Page 1
Only use the device after reading this manual.
CONTENTS
1. INTRODUCTION .............................................................................................................................................................. 2
1.1 W
HAT IS THE
I-ONE
AND HOW DOES IT WORK
?............................................................................................................................. 2
1.2 W
HO CAN USE THE
I-ONE? ....................................................................................................................................................... 2
1.3 I
NTENDED
U
SE
......................................................................................................................................................................... 2
1.4 D
EVICE PERFORMANCE CHARACTERISTICS
...................................................................................................................................... 2
1.5 T
REATMENTS THAT CAN BE CARRIED OUT WITH
I-ONE
T
HERAPY
....................................................................................................... 2
1.6 E
XPECTED CLINICAL BENEFITS
...................................................................................................................................................... 3
2. I-ONE DEVICE COMPONENTS .......................................................................................................................................... 3
2.1 G
ENERATOR
............................................................................................................................................................................ 3
3. DEVICE PREPARATION .................................................................................................................................................... 4
3.1 I
NITIAL BATTERY CHARGE
........................................................................................................................................................... 4
3.2 C
ONNECTING THE COIL TO THE GENERATOR
................................................................................................................................... 5
4. CLIP ATTACHMENT AND REMOVAL ................................................................................................................................ 5
5. ADMINISTERING THE TREATMENT.................................................................................................................................. 6
5.1 C
OIL POSITIONING
.................................................................................................................................................................... 6
5.2 T
URNING ON THE GENERATOR
.................................................................................................................................................... 6
5.3 B
ATTERY MONITORING AND CHARGING
......................................................................................................................................... 8
5.4 S
TATUS INDICATION OF THE DEVICE
.............................................................................................................................................. 8
5.5 B
ATTERY EFFICIENCY
............................................................................................................................................................... 10
5.6 T
REATMENT TIMES
................................................................................................................................................................. 10
5.7 U
SEFUL
T
IPS
......................................................................................................................................................................... 10
5.8 C
LEANING THE DEVICE
............................................................................................................................................................. 11
6. PROBLEM SOLVING ...................................................................................................................................................... 11
6.1 E
RROR MESSAGES
.................................................................................................................................................................. 11
6.2 A
NOMALIES OR BLOCKED DEVICE
............................................................................................................................................... 12
6.2.1 The device will not switch on and will not charge....................................................................................................... 12
6.2.2 Blocked device during normal operation. ................................................................................................................... 12
6.2.3 Technical Support ....................................................................................................................................................... 12
7. SAFETY INSTRUCTIONS ................................................................................................................................................. 12
7.1 W
ARNINGS AND
R
ECOMMENDATIONS
....................................................................................................................................... 12
7.2 M
AINTENANCE
...................................................................................................................................................................... 13
7.3 C
ONTRAINDICATIONS AND SIDE EFFECTS
..................................................................................................................................... 13
7.4 E
LECTROMAGNETIC
C
OMPATIBILITY
........................................................................................................................................... 14
7.5 B
IOLOGICAL SAFETY
................................................................................................................................................................ 14
8. MANUFACTURER’S LIABILITY ........................................................................................................................................ 14
9. DEVICE RETURNS .......................................................................................................................................................... 15
10. TECHNICAL DATA.......................................................................................................................................................... 15
10.1 T
ABLE
1
-
E
LECTROMAGNETIC
E
MISSIONS
................................................................................................................................ 16
10.2 T
ABLE
2
-
E
LECTROMAGNETIC
I
MMUNITY
................................................................................................................................ 16
10.3 I
MMUNITY TO PROXIMITY FIELDS FROM
RF
WIRELESS COMMUNICATION DEVICES
............................................................................. 17
10.4 I
NFORMATION PLATE
........................................................................................................................................................... 19
11. SYMBOLS...................................................................................................................................................................... 20
MI-IONE-EN Revision 1.4 - June 2023
SW Rev. from 1.6
CONTENTS
1. INTRODUCTION ................................................................................................................................................................. 3
1.1WHATISTHEI-ONEANDHOWDOESITWORK? ................................................................................................................................................ 3
1.2WHOCANUSETHEI-ONE?................................................................................................................................................................................... 3
1.3INTENDEDUSE...................................................................................................................................................................................................... 3
1.4DEVICEPERFORMANCECHARACTERISTICS....................................................................................................................................................... 3
1.5TREATMENTSTHATCANBECARRIEDOUTWITHI-ONETHERAPY ................................................................................................................... 3
1.6EXPECTEDCLINICALBENEFITS............................................................................................................................................................................ 4
2.I-ONEDEVICECOMPONENTS............................................................................................................................................4
2.1GENERATOR ........................................................................................................................................................................................................... 4
3.DEVICEPREPARATION ....................................................................................................................................................... 5
3.1INITIALBATTERYCHARGE.................................................................................................................................................................................... 5
3.2CONNECTINGTHECOILTOTHEGENERATOR..................................................................................................................................................... 6
4.CLIPATTACHMENTANDREMOVAL ...................................................................................................................................6
5.ADMINISTERINGTHETREATMENT.................................................................................................................................... 7
5.1COILPOSITIONING................................................................................................................................................................................................ 7
5.2TURNINGONTHEGENERATOR............................................................................................................................................................................ 7
5.3BATTERYMONITORINGANDCHARGING ............................................................................................................................................................ 9
5.4STATUSINDICATIONOFTHEDEVICE ................................................................................................................................................................... 9
5.5BATTERYEFFICIENCY........................................................................................................................................................................................... 11
5.6TREATMENTTIMES ............................................................................................................................................................................................. 11
5.7USEFULTIPS......................................................................................................................................................................................................... 11
5.8CLEANINGTHEDEVICE ....................................................................................................................................................................................... 12
6.PROBLEMSOLVING.......................................................................................................................................................... 12
6.1ERRORMESSAGES............................................................................................................................................................................................... 12
6.2ANOMALIESORBLOCKEDDEVICE..................................................................................................................................................................... 13
6.2.1 The device will not switch on and will not charge............................................................................................................................................. 13
6.2.2Blockeddeviceduringnormaloperaon........................................................................................................................................................... 13
6.2.3 Technical Support .............................................................................................................................................................................................. 13
7.SAFETYINSTRUCTIONS.................................................................................................................................................... 13
7.1WARNINGSANDRECOMMENDATIONS............................................................................................................................................................ 13
7.2MAINTENANCE.................................................................................................................................................................................................... 14
7.3CONTRAINDICATIONSANDSIDEEFFECTS ........................................................................................................................................................ 14
7.4ELECTROMAGNETICCOMPATIBILITY................................................................................................................................................................ 15
7.5BIOLOGICALSAFETY........................................................................................................................................................................................... 15
8.MANUFACTURER’SLIABILITY .......................................................................................................................................... 15
9.DEVICERETURNS ............................................................................................................................................................. 16
10.TECHNICALDATA........................................................................................................................................................... 16
10.1TABLE1-ELECTROMAGNETICEMISSIONS ..................................................................................................................................................... 17
10.2TABLE2-ELECTROMAGNETICIMMUNITY..................................................................................................................................................... 17
10.3IMMUNITYTOPROXIMITYFIELDSFROMRFWIRELESSCOMMUNICATIONDEVICES ............................................................................... 18
10.4INFORMATIONPLATE........................................................................................................................................................................................................................ 20
11.SYMBOLS........................................................................................................................................................................ 21
IGEA I-ONE - User Manual
Page 1
Only use the device after reading this manual.
CONTENTS
1. INTRODUCTION .............................................................................................................................................................. 2
1.1 W
HAT IS THE
I-ONE
AND HOW DOES IT WORK
?............................................................................................................................. 2
1.2 W
HO CAN USE THE
I-ONE? ....................................................................................................................................................... 2
1.3 I
NTENDED
U
SE
......................................................................................................................................................................... 2
1.4 D
EVICE PERFORMANCE CHARACTERISTICS
...................................................................................................................................... 2
1.5 T
REATMENTS THAT CAN BE CARRIED OUT WITH
I-ONE
T
HERAPY
....................................................................................................... 2
1.6 E
XPECTED CLINICAL BENEFITS
...................................................................................................................................................... 3
2. I-ONE DEVICE COMPONENTS .......................................................................................................................................... 3
2.1 G
ENERATOR
............................................................................................................................................................................ 3
3. DEVICE PREPARATION .................................................................................................................................................... 4
3.1 I
NITIAL BATTERY CHARGE
........................................................................................................................................................... 4
3.2 C
ONNECTING THE COIL TO THE GENERATOR
................................................................................................................................... 5
4. CLIP ATTACHMENT AND REMOVAL ................................................................................................................................ 5
5. ADMINISTERING THE TREATMENT.................................................................................................................................. 6
5.1 C
OIL POSITIONING
.................................................................................................................................................................... 6
5.2 T
URNING ON THE GENERATOR
.................................................................................................................................................... 6
5.3 B
ATTERY MONITORING AND CHARGING
......................................................................................................................................... 8
5.4 S
TATUS INDICATION OF THE DEVICE
.............................................................................................................................................. 8
5.5 B
ATTERY EFFICIENCY
............................................................................................................................................................... 10
5.6 T
REATMENT TIMES
................................................................................................................................................................. 10
5.7 U
SEFUL
T
IPS
......................................................................................................................................................................... 10
5.8 C
LEANING THE DEVICE
............................................................................................................................................................. 11
6. PROBLEM SOLVING ...................................................................................................................................................... 11
6.1 E
RROR MESSAGES
.................................................................................................................................................................. 11
6.2 A
NOMALIES OR BLOCKED DEVICE
............................................................................................................................................... 12
6.2.1 The device will not switch on and will not charge....................................................................................................... 12
6.2.2 Blocked device during normal operation. ................................................................................................................... 12
6.2.3 Technical Support ....................................................................................................................................................... 12
7. SAFETY INSTRUCTIONS ................................................................................................................................................. 12
7.1 W
ARNINGS AND
R
ECOMMENDATIONS
....................................................................................................................................... 12
7.2 M
AINTENANCE
...................................................................................................................................................................... 13
7.3 C
ONTRAINDICATIONS AND SIDE EFFECTS
..................................................................................................................................... 13
7.4 E
LECTROMAGNETIC
C
OMPATIBILITY
........................................................................................................................................... 14
7.5 B
IOLOGICAL SAFETY
................................................................................................................................................................ 14
8. MANUFACTURER’S LIABILITY ........................................................................................................................................ 14
9. DEVICE RETURNS .......................................................................................................................................................... 15
10. TECHNICAL DATA.......................................................................................................................................................... 15
10.1 T
ABLE
1
-
E
LECTROMAGNETIC
E
MISSIONS
................................................................................................................................ 16
10.2 T
ABLE
2
-
E
LECTROMAGNETIC
I
MMUNITY
................................................................................................................................ 16
10.3 I
MMUNITY TO PROXIMITY FIELDS FROM
RF
WIRELESS COMMUNICATION DEVICES
............................................................................. 17
10.4 I
NFORMATION PLATE
........................................................................................................................................................... 19
11. SYMBOLS...................................................................................................................................................................... 20
MI-IONE-EN Revision 1.4 - June 2023
SW Rev. from 1.6
IGEA I-ONE - User Manual
Page 2
1. INTRODUCTION
1.1 What is the I-ONE and how does it work?
The I-ONE is a medical device for the treatment of inflammatory and degenerative tissue diseases using low-
frequency electromagnetic fields.
The I-ONE is a therapeutic aid and must be used under a doctor's prescription.
The device consists of a low-frequency pulsed electromagnetic field generator characterised by a pulse signal at a
frequency of 75 Hz, with a pulse width (trigger time) of approximately 1.0 millisecond.
This electromagnetic field is capable of inducing an average electric field of 0.04 mV/cm in the bone tissue, which
represents the active component of the signal and is capable of increasing osteoblast activity.
The electric field focusses on the site to be treated by means of appropriately shaped coils.
The generator is controlled by a microprocessor that constantly monitors the correct functioning of the device,
promptly signalling any anomalies or malfunctions to the patient that may occur during the treatment; to this end,
it is equipped with simple and effective visual and acoustic alarms.
1.2 Who can use the I-ONE?
The I-ONE must be used by people who are capable of independently understanding and implementing the
instructions provided in this manual; otherwise, and if it is being used on children, the I-ONE may only be used under
the supervision of people who are capable of understanding and implementing the instructions provided in this
manual.
1.3 Intended Use
The intended use of the low frequency pulsed electromagnetic field generator for therapeutic use is the treatment
of inflammatory and degenerative tissue diseases, with particular reference to the joints and the stimulation of
osteogenesis. In particular, the I-ONE device, model CBA04 is indicated for:
• Treatment of inflammatory and degenerative tissue diseases
1.4 Device performance characteristics
The device performance characteristics are:
- the ability to generate an electrical signal with the specified characteristics capable of driving a coil and
producing a pulsed electromagnetic field that provides the expected clinical benefits;
- the device must allow the user to activate/deactivate the signal delivery and check the time the treatment
is performed at.
More specifically, the time-varying electromagnetic field generated produces a specific effect on the receptors that
control inflammation. The effect on inflammation, which is related to the agonist activity of adenosine for A2A
receptors, justifies the indication for use in various tissues.
1.5 Treatments that can be carried out with I-ONE Therapy
The main indications for which the I-ONE is used are:
- Ligament reconstruction
- Microfractures of the subchondral bone
- Joint fractures
- Joint inflammatory processes
- Oedema
- Autologous chondrocyte grafts
- Osteochondral grafts
- Early stages of arthrosis
- Meniscectomy
- Algodystrophy
- Knee prosthesis
- Femoro-rotulea syndrome
The coil to be applied to the treatment site has a homogeneous field and does not require a perfectly centred
application on the site to be treated; for this reason the patient is able to perform the application independently,
2

ENGLISH
IGEA I-ONE - User Manual
Page 2
1. INTRODUCTION
1.1 What is the I-ONE and how does it work?
The I-ONE is a medical device for the treatment of inflammatory and degenerative tissue diseases using low-
frequency electromagnetic fields.
The I-ONE is a therapeutic aid and must be used under a doctor's prescription.
The device consists of a low-frequency pulsed electromagnetic field generator characterised by a pulse signal at a
frequency of 75 Hz, with a pulse width (trigger time) of approximately 1.0 millisecond.
This electromagnetic field is capable of inducing an average electric field of 0.04 mV/cm in the bone tissue, which
represents the active component of the signal and is capable of increasing osteoblast activity.
The electric field focusses on the site to be treated by means of appropriately shaped coils.
The generator is controlled by a microprocessor that constantly monitors the correct functioning of the device,
promptly signalling any anomalies or malfunctions to the patient that may occur during the treatment; to this end,
it is equipped with simple and effective visual and acoustic alarms.
1.2 Who can use the I-ONE?
The I-ONE must be used by people who are capable of independently understanding and implementing the
instructions provided in this manual; otherwise, and if it is being used on children, the I-ONE may only be used under
the supervision of people who are capable of understanding and implementing the instructions provided in this
manual.
1.3 Intended Use
The intended use of the low frequency pulsed electromagnetic field generator for therapeutic use is the treatment
of inflammatory and degenerative tissue diseases, with particular reference to the joints and the stimulation of
osteogenesis. In particular, the I-ONE device, model CBA04 is indicated for:
• Treatment of inflammatory and degenerative tissue diseases
1.4 Device performance characteristics
The device performance characteristics are:
- the ability to generate an electrical signal with the specified characteristics capable of driving a coil and
producing a pulsed electromagnetic field that provides the expected clinical benefits;
- the device must allow the user to activate/deactivate the signal delivery and check the time the treatment
is performed at.
More specifically, the time-varying electromagnetic field generated produces a specific effect on the receptors that
control inflammation. The effect on inflammation, which is related to the agonist activity of adenosine for A2A
receptors, justifies the indication for use in various tissues.
1.5 Treatments that can be carried out with I-ONE Therapy
The main indications for which the I-ONE is used are:
- Ligament reconstruction
- Microfractures of the subchondral bone
- Joint fractures
- Joint inflammatory processes
- Oedema
- Autologous chondrocyte grafts
- Osteochondral grafts
- Early stages of arthrosis
- Meniscectomy
- Algodystrophy
- Knee prosthesis
- Femoro-rotulea syndrome
The coil to be applied to the treatment site has a homogeneous field and does not require a perfectly centred
application on the site to be treated; for this reason the patient is able to perform the application independently,
3

ENGLISH
IGEA I-ONE - User Manual
Page 3
without the need for medical or nursing supervision. The device operates on a rechargeable battery with an external
power supply.
1.6 Expected clinical benefits
The expected clinical benefits of using the I-ONE CBA04 pulsed electromagnetic field generator are:
• Chondroprotection,
• pain relief,
• functional recovery,
• a better quality of life.
These clinical benefits and claims of product performance are reported in multiple scientific articles and confirmed
by clinical trials.
2. I-ONE DEVICE COMPONENTS
The I-ONE device consists of the following
components:
①The generator that incorporates the
rechargeable battery
②The coil, the applied part of the device
③The external power supply unit
④An elastic band to keep the coil in the
correct position during the treatment
2.1 Generator
The generator is equipped with:
(A) A display with a touch-screen function that
shows the status of the device and allows certain
functions to be activated by pressing the button
shown on the display
(B) A multifunctional button for switching the
device on/off/reset
(C) A multi-coloured LED whose activation and
colour indicates the status of the device in addition to
the messages on the display.
(D) A coil connection socket, marked with the
symbol
(E) A socket for connection to the external power supply, marked with the symbol
(F) A service socket at the bottom of the generator which is reserved for technical support.
(G) A removable attachment clip that the user can use to carry the generator on a belt and perform the treatment
on the move.
①
②
③
A
B
C
D
E
F
④
G
IGEA I-ONE - User Manual
Page 4
3. DEVICE PREPARATION
3.1 Initial battery charge
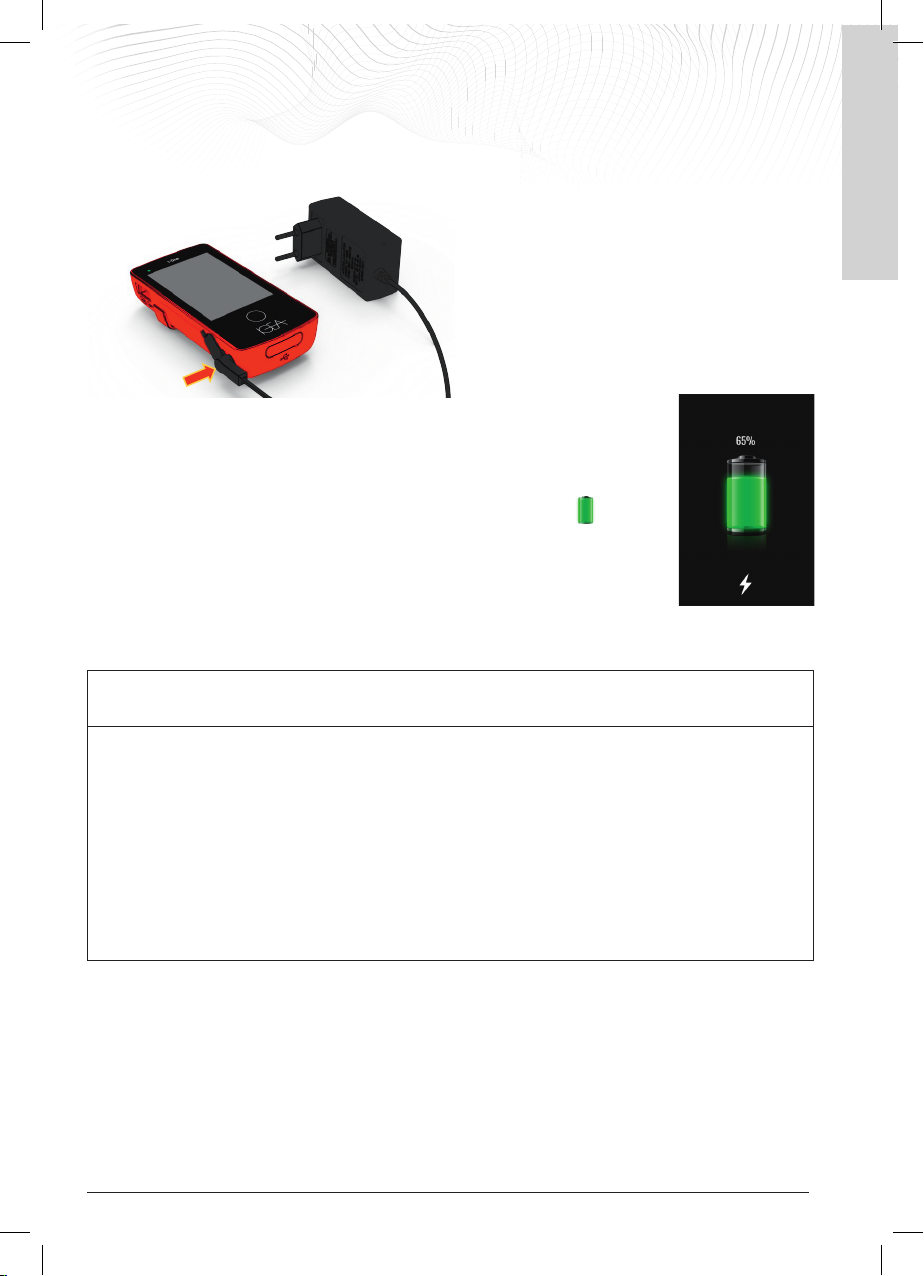
Upon receipt, the battery must be charged before treatment using the external power supply.
With the generator switched off, connect the power
supply to the generator by inserting connector [A] into
the socket on the bottom left of the generator. Then
connect external power supply plug [B] to the mains
socket.
Within a few seconds, the device will start to charge the
battery:
The generator beeps, the display lights up and shows a
progressively filling battery symbol to indicate that the
battery is charging.
The charge percentage also
appears above the battery symbol.
- charging a completely empty battery can take up to 3 hours
- it is a normal for the device to heat up when charging and it should not be cause for concern
- when charging is complete, the display shows the battery charged symbol.
Disconnect the power supply unit from the generator and the mains socket.
NOTE on the room temperature: if the I-ONE is charged in an environment with a
temperature above 30 °C, the battery may take longer than 3 hours to fully charge.
To avoid this, the I-ONE should be charged in an environment with a temperature that does
not exceed 30 °C, where possible.
IMPORTANT
Only use the supplied power supply unit for charging. The use of other devices may cause damage to the device
or the user and the manufacturer waives any liability for this.
If the device comes from a place with a different temperature (e.g. due to transportation or storage), wait about
10 minutes for it to adjust to the room temperature before using it.
If the device is not used for long periods, the battery may go flat or may not have enough energy left to complete
the treatment; it is therefore recommended to charge the battery before each use.
1. Under special conditions, e.g. after long periods of storage or inactivity, the battery may be completely
flat and the device may not be able to switch on; in this case, connect the external power supply to the
generator and wait up to 30 seconds; the battery should begin to charge as described in section 3.1.
2. Leave the generator on charge until it is fully charged before using the device.
3. The power supply battery contained in the device cannot be removed/replaced by the user. The battery
may only be replaced, if necessary, by the manufacturer or by the manufacturer's authorised technical
support.
A
B
4
ENGLISH
IGEA I-ONE - User Manual
Page 4
3. DEVICE PREPARATION
3.1 Initial battery charge
Upon receipt, the battery must be charged before treatment using the external power supply.
With the generator switched off, connect the power
supply to the generator by inserting connector [A] into
the socket on the bottom left of the generator. Then
connect external power supply plug [B] to the mains
socket.
Within a few seconds, the device will start to charge the
battery:
The generator beeps, the display lights up and shows a
progressively filling battery symbol to indicate that the
battery is charging.
The charge percentage also
appears above the battery symbol.
- charging a completely empty battery can take up to 3 hours
- it is a normal for the device to heat up when charging and it should not be cause for concern
- when charging is complete, the display shows the battery charged symbol.
Disconnect the power supply unit from the generator and the mains socket.
NOTE on the room temperature: if the I-ONE is charged in an environment with a
temperature above 30 °C, the battery may take longer than 3 hours to fully charge.
To avoid this, the I-ONE should be charged in an environment with a temperature that does
not exceed 30 °C, where possible.
IMPORTANT
Only use the supplied power supply unit for charging. The use of other devices may cause damage to the device
or the user and the manufacturer waives any liability for this.
If the device comes from a place with a different temperature (e.g. due to transportation or storage), wait about
10 minutes for it to adjust to the room temperature before using it.
If the device is not used for long periods, the battery may go flat or may not have enough energy left to complete
the treatment; it is therefore recommended to charge the battery before each use.
1. Under special conditions, e.g. after long periods of storage or inactivity, the battery may be completely
flat and the device may not be able to switch on; in this case, connect the external power supply to the
generator and wait up to 30 seconds; the battery should begin to charge as described in section 3.1.
2. Leave the generator on charge until it is fully charged before using the device.
3. The power supply battery contained in the device cannot be removed/replaced by the user. The battery
may only be replaced, if necessary, by the manufacturer or by the manufacturer's authorised technical
support.
A
B
5
ENGLISH
IGEA I-ONE - User Manual
Page 5
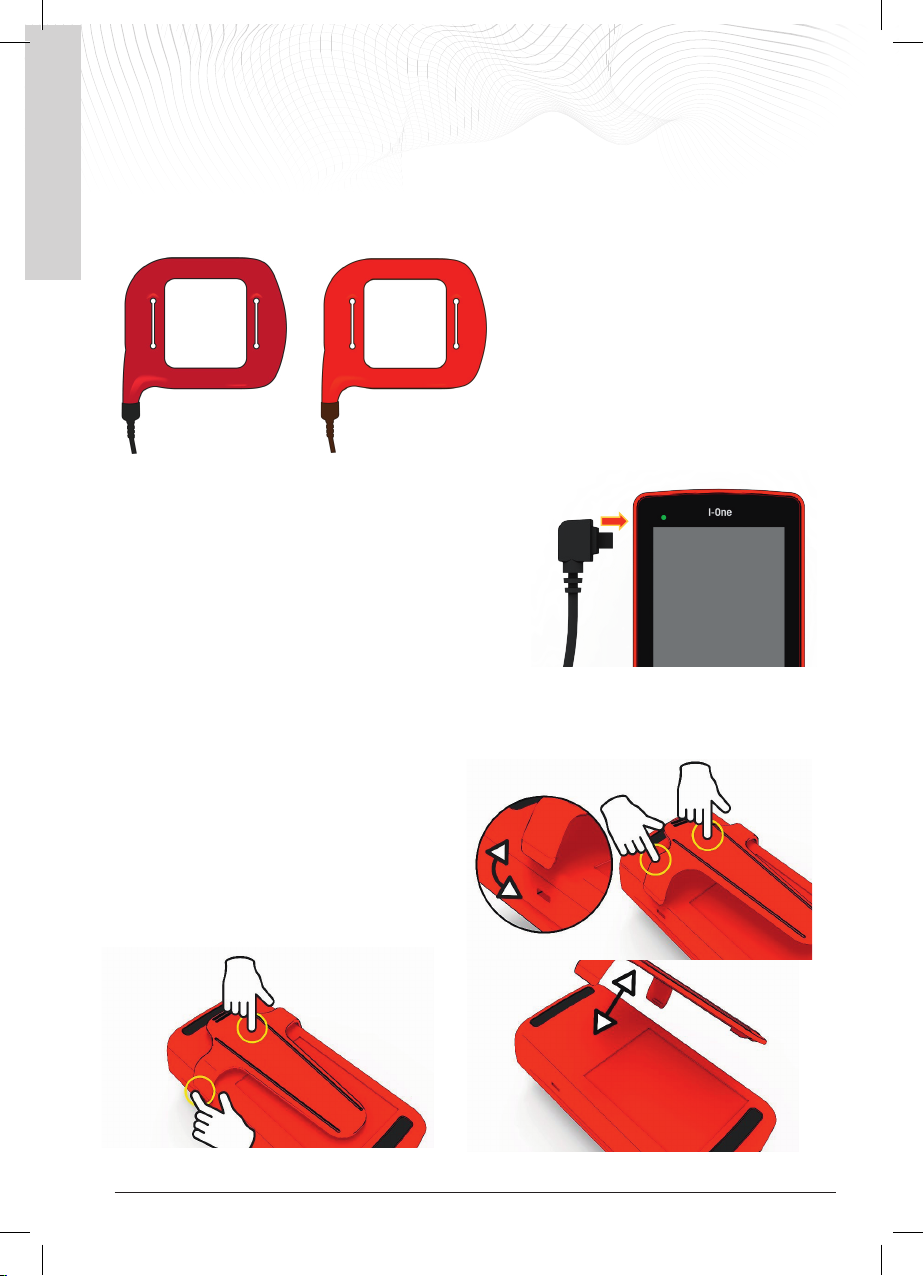
3.2 Connecting the coil to the generator
The coil that is compatible with the I-ONE is shown in the figure below, identified by its code:
REF. 70010
REF. 70110
Insert the coil connector into the coil socket on the left side of the
generator (P), pressing down until you hear a 'click'.
When switched on, the device will automatically recognise the
connected coil and emit the required alarm level.
4. CLIP ATTACHMENT AND REMOVAL
If necessary, the generator can be clipped onto the belt using the supplied clip, so that the treatment can also be
carried out on the move.
To fit the clip, engage one side notch in the slot provided
and apply light pressure to the centre of the clip until the
second notch is also fully engaged.
The generator can now be attached to the belt.
To remove the clip, press gently in the centre to release
the first side notch from its socket, followed by the second.
Now lift the clip and remove it.
P
IGEA I-ONE - User Manual
Page 6
5. ADMINISTERING THE TREATMENT
5.1 Coil positioning
Position the coil so that the place undergoing treatment is in the centre of the coil,
then secure it with the supplied band or other suitable means; an example of
positioning it on the knee is shown here.
It is not necessary for the coil to come into contact with the skin; for hygienic
reasons, it is always recommended to place the coil on light clothing, the presence
of which does not have an impact on the treatment. Particularly if the skin has
lesions in the treatment area, place a light garment between the coil and the skin
or, if this is not possible, clean the coil before each application.
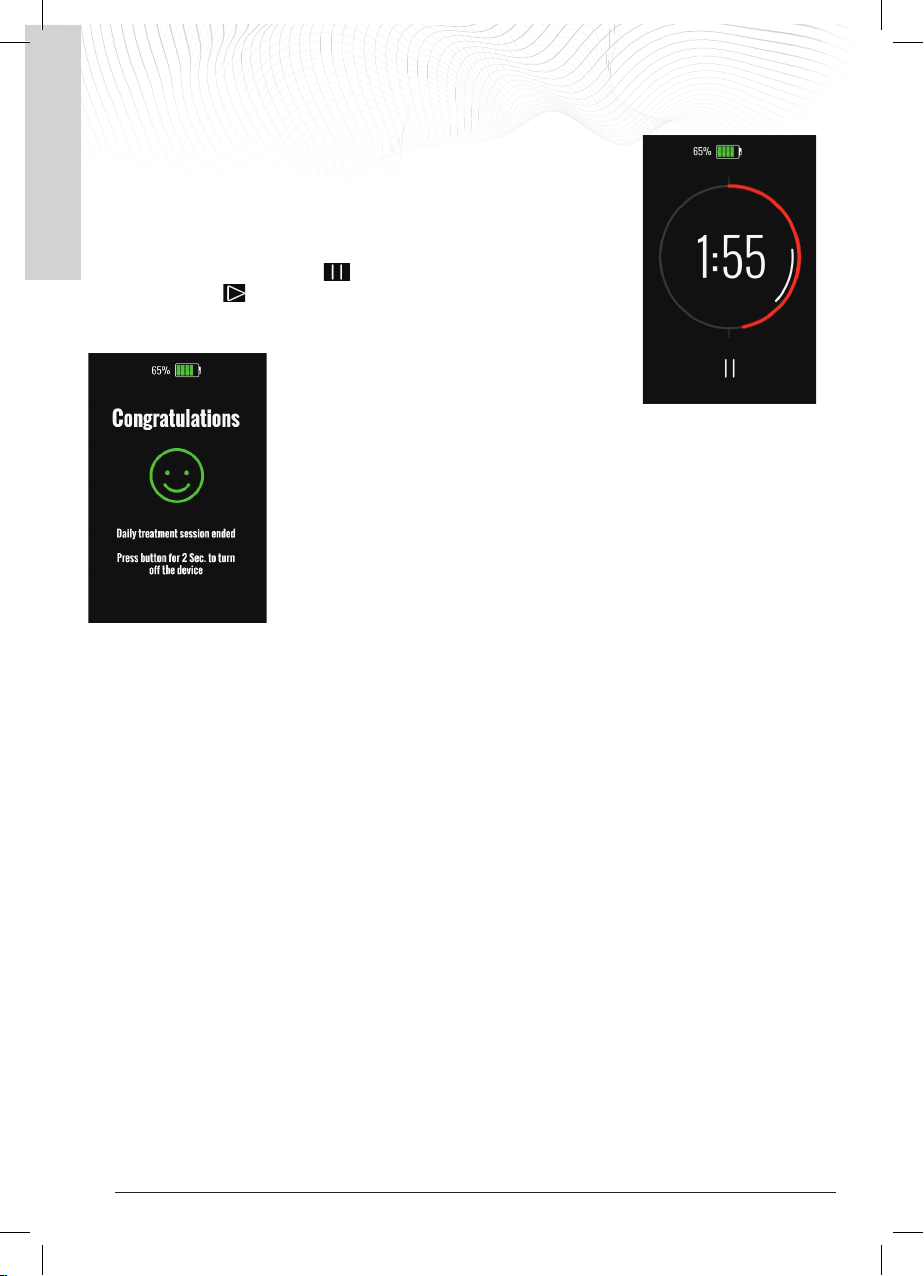
5.2 Turning on the generator
After charging the battery and connecting the coil, switch on the generator by
pressing the power button for about 2 seconds until you hear a confirmation 'beep'
and a short vibration, then release the button.
The display lights up and an initial Welcome screen appears. Then the main screen
appears with the battery symbol at the top and the timer counting down the treatment time in the middle of the
screen. The device immediately starts providing the treatment. Below the timer, the PAUSE button allows the
treatment to be paused.
The
I-ONE suggests a treatment time of 4 hours every time it is switched on, which is
the recommended time in order for the treatment to be effective.
The LED at the top of the display flashes green and the timer on the display starts to
count down the treatment time.
In the adjacent figure you can see the timer, where 3:00 is the remaining treatment
time. The timer updates with every minute of treatment that has been performed until
the preset daily treatment time of 4 hours is reached.
During the treatment phase, it must be remembered that:
After 10 seconds of inactivity, the display lowers its brightness, and after 30 seconds, it
switches off to save battery power; the green LED that continues to flash informs the
patient that treatment is in progress.
During treatment, the patient can reactivate the display
with a quick press of the ON/OFF button, e.g. to read the treatment time remaining
or the remaining battery charge.
When switched on, the display shows:
ON/OFF
button
6

ENGLISH
IGEA I-ONE - User Manual
Page 6
5. ADMINISTERING THE TREATMENT
5.1 Coil positioning
Position the coil so that the place undergoing treatment is in the centre of the coil,
then secure it with the supplied band or other suitable means; an example of
positioning it on the knee is shown here.
It is not necessary for the coil to come into contact with the skin; for hygienic
reasons, it is always recommended to place the coil on light clothing, the presence
of which does not have an impact on the treatment. Particularly if the skin has
lesions in the treatment area, place a light garment between the coil and the skin
or, if this is not possible, clean the coil before each application.
5.2 Turning on the generator
After charging the battery and connecting the coil, switch on the generator by
pressing the power button for about 2 seconds until you hear a confirmation 'beep'
and a short vibration, then release the button.
The display lights up and an initial Welcome screen appears. Then the main screen
appears with the battery symbol at the top and the timer counting down the treatment time in the middle of the
screen. The device immediately starts providing the treatment. Below the timer, the PAUSE button allows the
treatment to be paused.
The
I-ONE suggests a treatment time of 4 hours every time it is switched on, which is
the recommended time in order for the treatment to be effective.
The LED at the top of the display flashes green and the timer on the display starts to
count down the treatment time.
In the adjacent figure you can see the timer, where 3:00 is the remaining treatment
time. The timer updates with every minute of treatment that has been performed until
the preset daily treatment time of 4 hours is reached.
During the treatment phase, it must be remembered that:
After 10 seconds of inactivity, the display lowers its brightness, and after 30 seconds, it
switches off to save battery power; the green LED that continues to flash informs the
patient that treatment is in progress.
During treatment, the patient can reactivate the display
with a quick press of the ON/OFF button, e.g. to read the treatment time remaining
or the remaining battery charge.
When switched on, the display shows:
ON/OFF
button
7
ENGLISH
IGEA I-ONE - User Manual
Page 7
The battery symbol above indicating the percentage of charge remaining; it is normally green and turns red when
the battery is low (the battery needs to be charged).
In the centre of the display, the timer symbol shows the treatment time that is left.
Initially, the circle containing the timer is grey and turns red as the patient performs
the treatment. The circle will go completely red at the end of the planned 4 hours of
treatment (the treatment cycle has been completed).
The rotating white bar inside the timer indicates that treatment is in progress.
Below the timer is the PAUSE button which, when pressed, pauses the treatment
and the PLAY button appears on the screen in its place. Pressing the button again
restarts the treatment and the treatment time countdown. Each press of the
PLAY/PAUSE button is accompanied by a confirmation beep.
At the end of the daily treatment time of 4 hours, the I-
ONE stops providing the treatment, the green light
goes out and the display shows the end of treatment
message.
The I-ONE remains switched on, without providing the treatment, in standby position.
The user can switch it off by pressing the OFF button for about two seconds, until
there is a beep and a short confirmation vibration.
If not switched off by the user, the I-ONE will switch itself off when the battery is low.
At the end of the treatment, remove the coil from the treatment area, keeping the
coil connected to the I-ONE for convenience.
Every time it is turned on, the timer will restart at 4:00 hours.
If the user has to interrupt treatment before having completed the set daily treatment
time, they can put the I-ONE on PAUSE and resume treatment later by pressing the
PLAY button. This can be done even several hours later. In this case, the treatment counter will restart from the
remaining time it showed when it was put on PAUSE.
Alternatively, the patient can switch the I-ONE off by pressing the ON/OFF button for about two seconds, until the
confirmation beep is heard.
To resume treatment, the patient must switch the I-ONE back on; in this case the treatment timer will reset to the
initial 4-hour value.
When the battery is fully charged, it allows up to 4 hours of continuous treatment.
The device should always be charged at the end of the daily treatment so that, the next time it is used, the device
is charged and able to provide the full treatment cycle.
NOTE: if the user wants to perform treatment for more than 4 hours each day, when the first 4 hours have been
reached and the end of treatment message appears, they must switch the device off and then switch it on again to
start a new treatment cycle. However, please note that:
- clinical studies have shown that treatment with the I-ONE is effective when performed for 4 hours a day
- the battery provides up to 4 hours of continuous treatment.
For more than 4 hours, if the battery runs out, the external power supply must be connected while the I-ONE is in
operation. This way, the I-ONE provides the treatment and, at the same time, charges the battery (see section 5.3).
At the end of treatment, leave the I-ONE switched off and connected to the power supply until the battery is fully
charged.
IGEA I-ONE - User Manual
Page 8
5.3 Battery monitoring and charging
The I-ONE can also charge the battery during treatment.
In the top of the display, the battery symbol is always shown indicating the percentage
of remaining charge. The symbol is normally green and turns red when the battery
needs charging.
If the external charger is connected during treatment, a flash appears inside the battery
symbol to indicate that charging is in progress, and there is a beep as a warning that the
power supply is being switched on.
With the generator switched off, to charge the battery, connect the power supply first
to the generator and then to the mains socket.
• The display lights up and the generator beeps and
vibrates.
• The display shows the animation of the battery being
charged and a flash symbol below it
• The animation persists until the charge is complete (full charge may take up
to 3 hours)
• When charging is complete, the display shows the battery charge symbol →
• Disconnect the power supply unit from the generator and the mains socket.
If the I-ONE is switched on during charging, pressing the power button turns on the
generator and the user can continue with the treatment whilst the battery is being
charged. Under these conditions, the device behaves as described in section 5.2.
At the end of treatment, leave the I-ONE switched off and connected to the power supply
until the battery charge is complete.
As it is normal for the battery to heat up when charging, if treatment is to be carried out whilst charging, it is
recommended not to place the I-ONE in direct contact with the body.
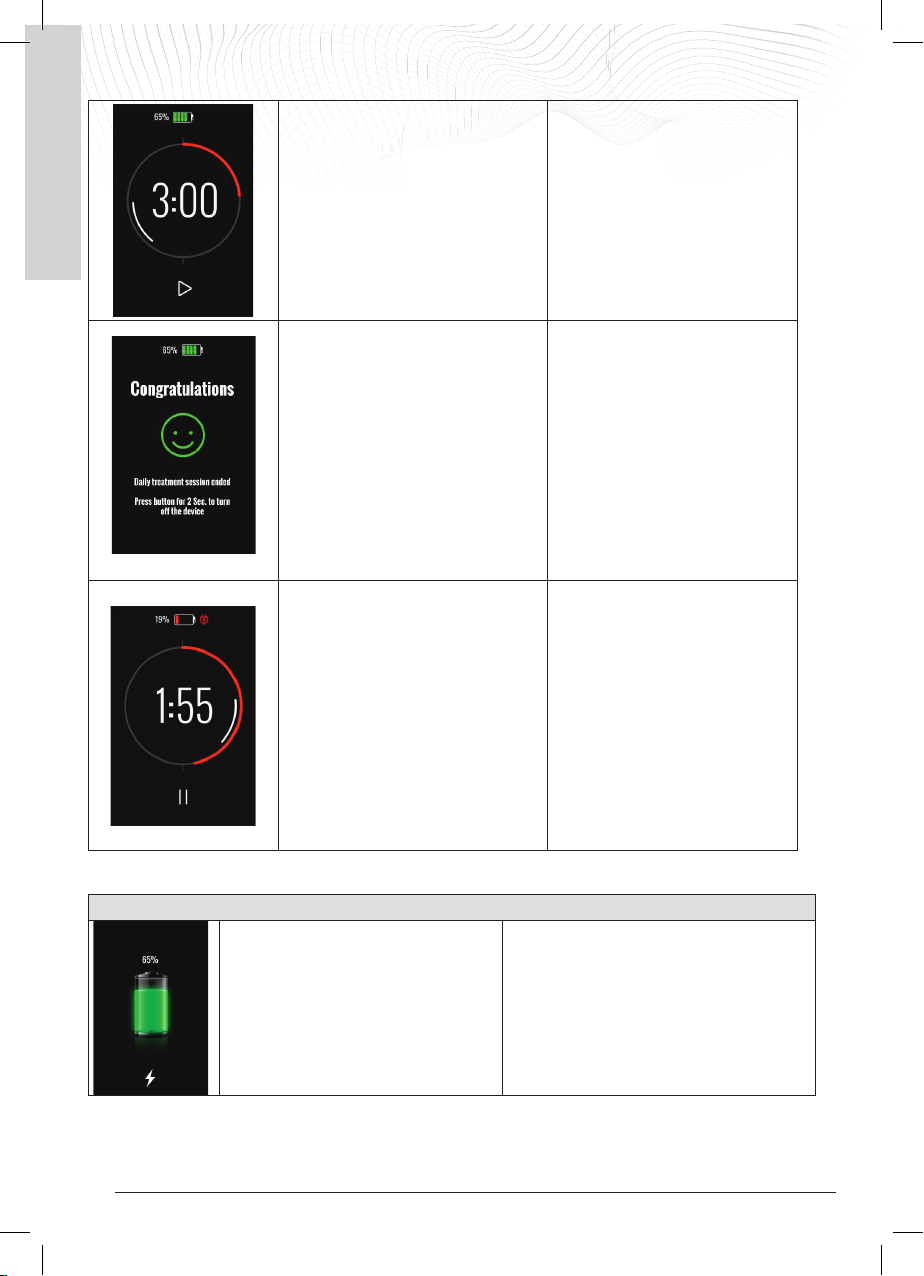
5.4 Status indication of the device
Generator on during treatment
Display
LED and alarms
Device status
The LED at the top is ON with a flashing
green light
The timer appears on the display with the
treatment time decreasing every minute
and the white bar rotates inside the
timer.
The PAUSE symbol is visible below the
timer. Pressing it interrupts the
treatment delivery.
The I-ONE is switched on and is
providing the treatment.
The battery symbol in the top centre
shows the percentage of charge
remaining; it is green up to 20% and
red when the capacity is > 20%.
8

ENGLISH
IGEA I-ONE - User Manual
Page 8
5.3 Battery monitoring and charging
The I-ONE can also charge the battery during treatment.
In the top of the display, the battery symbol is always shown indicating the percentage
of remaining charge. The symbol is normally green and turns red when the battery
needs charging.
If the external charger is connected during treatment, a flash appears inside the battery
symbol to indicate that charging is in progress, and there is a beep as a warning that the
power supply is being switched on.
With the generator switched off, to charge the battery, connect the power supply first
to the generator and then to the mains socket.
• The display lights up and the generator beeps and
vibrates.
• The display shows the animation of the battery being
charged and a flash symbol below it
• The animation persists until the charge is complete (full charge may take up
to 3 hours)
• When charging is complete, the display shows the battery charge symbol →
• Disconnect the power supply unit from the generator and the mains socket.
If the I-ONE is switched on during charging, pressing the power button turns on the
generator and the user can continue with the treatment whilst the battery is being
charged. Under these conditions, the device behaves as described in section 5.2.
At the end of treatment, leave the I-ONE switched off and connected to the power supply
until the battery charge is complete.
As it is normal for the battery to heat up when charging, if treatment is to be carried out whilst charging, it is
recommended not to place the I-ONE in direct contact with the body.
5.4 Status indication of the device
Generator on during treatment
Display
LED and alarms
Device status
The LED at the top is ON with a flashing
green light
The timer appears on the display with the
treatment time decreasing every minute
and the white bar rotates inside the
timer.
The PAUSE symbol is visible below the
timer. Pressing it interrupts the
treatment delivery.
The I-ONE is switched on and is
providing the treatment.
The battery symbol in the top centre
shows the percentage of charge
remaining; it is green up to 20% and
red when the capacity is > 20%.
9
ENGLISH
IGEA I-ONE - User Manual
Page 9
The green LED stays on to indicate that
the I-ONE is on PAUSE
The timer with the set treatment time
appears on the display and the white bar
inside the timer disappears. The PLAY
symbol is visible below the timer.
Pressing it resumes treatment.
The I-ONE is switched on but is on
PAUSE. To resume treatment, press
the PLAY symbol on the display.
The I-ONE can also be left on PAUSE
for several hours, depending on the
initial battery status.
If the I-ONE is paused and the battery
goes low, a LOW BATTERY warning is
given.
The LED at the top is OFF
The completed treatment symbol
appears on the display.
The patient is asked to switch off the
generator by pressing the OFF button for
about 2 seconds.
Daily treatment was completed.
The device remains on standby and
emits no sounds or vibrations so as not
to disturb the user if it is on during the
night. With a quick press of the On/Off
button, the display lights up and the
patient is informed that the treatment
has finished. At this point, the device
should be switched off.
The LED at the top flashes green (the
treatment is in progress).
20% battery: The generator beeps, the
battery symbol turns RED and a power
plug symbol appears next to it.
If the battery is not charged:
5% battery: when the level drops to 5%,
the device interrupts the treatment. The
battery symbol flashes and the alarm
beeps every 5 seconds.
After 60 seconds, the I-ONE shuts down.
The I-ONE is providing the treatment
but the battery is almost empty and
needs to be charged.
The battery can be charged either
during treatment delivery or after
switching off the generator.
Connect the external power supply
and charge the battery.
Generator switched off during battery charging
When the external power supply is
connected, the generator beeps and
vibrates.
The display lights up and shows the image
of the battery being charged.
The charge percentage appears above the
battery symbol.
The device is charging the battery.
When the battery is completely empty, charging
takes 2 to 3 hours depending on the battery’s
initial status.
IGEA I-ONE - User Manual
Page 10
Connected power supply
The generator beeps and the
100% FULL battery symbol is displayed.
The battery is charged: disconnect the power
supply.
The device switches OFF when the power
supply is disconnected.
5.5 Battery efficiency
The efficiency of the battery is affected by the correct use and wear of the battery.
If the battery does not allow 4 consecutive hours of treatment, daily treatment can be performed using the external
power supply.
When the display indicates that the battery is running low, the battery symbol turns red and a plug symbol appears
next to it. Connect the power supply to the generator and to the mains socket, keeping the generator switched on:
the device beeps and vibrates, indicating that the charger has been connected to the generator. The plug symbol
disappears and a flash is displayed in its place, indicating that charging is in progress. The device continues to
administer the treatment while charging the battery. At the end of the treatment, leave the generator switched off
and connected to the power supply until the battery charge is complete.
5.6 Treatment times
The user must undergo the treatment for the number of days indicated by the prescribing doctor.
Clinical studies have shown that the I-ONE treatment is effective if performed for 4 hours a day; in any case, there
are no problems or side effects due to overdosing.
It is good practice to perform the daily treatment in a single session; however, it is possible to split the treatment
time into several daily applications of no less than 2 hours. The absence of side effects means that the treatment
can be carried out even whilst sleeping.
5.7 Useful Tips
• To make using the device easier, it is recommended to leave the coil connected to the generator to avoid
having to keep connecting it for each new treatment session.
• It is recommended that the battery be charged every day after the treatment is completed, so that the next
treatment can be fully performed. Please note that it is also possible to carry out the treatment using the
mains power by connecting the generator to the external power supply.
• The parts of the device that may come into contact with the skin do not normally cause any allergic reaction.
Although the coil's covering material is hypoallergenic and biocompatible, it is recommended not to place
the coil in direct contact with the skin, but to place it on a light garment, especially if there is any reddening
or irritation in the area of application.
• Clean the coil regularly, taking care to disconnect the coil from the generator using neutral detergents.
• Any wear on the coil coating due to use does not affect the effectiveness of the treatment. However, in the
event of a loss of some of the coating, the coil must be replaced.
• When using the coil under heavy blankets, the coil surface may become overheated. If this is the case,
perform the treatment without covering the coil.
• In any case, the generator must not be covered to allow ventilation whilst it is in operation.
• Using the device in environments with a temperature above 30°C, although possible, could cause the coil
surface to slightly overheat and this may be uncomfortable for the user.
• When charging or operating via the mains, it is normal for the battery to get hot; for this reason, the
generator should not be placed in direct contact with the body when charging or operating via the mains.
• The elastic band can be washed just like any other garment.
10

ENGLISH
IGEA I-ONE - User Manual
Page 10
Connected power supply
The generator beeps and the
100% FULL battery symbol is displayed.
The battery is charged: disconnect the power
supply.
The device switches OFF when the power
supply is disconnected.
5.5 Battery efficiency
The efficiency of the battery is affected by the correct use and wear of the battery.
If the battery does not allow 4 consecutive hours of treatment, daily treatment can be performed using the external
power supply.
When the display indicates that the battery is running low, the battery symbol turns red and a plug symbol appears
next to it. Connect the power supply to the generator and to the mains socket, keeping the generator switched on:
the device beeps and vibrates, indicating that the charger has been connected to the generator. The plug symbol
disappears and a flash is displayed in its place, indicating that charging is in progress. The device continues to
administer the treatment while charging the battery. At the end of the treatment, leave the generator switched off
and connected to the power supply until the battery charge is complete.
5.6 Treatment times
The user must undergo the treatment for the number of days indicated by the prescribing doctor.
Clinical studies have shown that the I-ONE treatment is effective if performed for 4 hours a day; in any case, there
are no problems or side effects due to overdosing.
It is good practice to perform the daily treatment in a single session; however, it is possible to split the treatment
time into several daily applications of no less than 2 hours. The absence of side effects means that the treatment
can be carried out even whilst sleeping.
5.7 Useful Tips
• To make using the device easier, it is recommended to leave the coil connected to the generator to avoid
having to keep connecting it for each new treatment session.
• It is recommended that the battery be charged every day after the treatment is completed, so that the next
treatment can be fully performed. Please note that it is also possible to carry out the treatment using the
mains power by connecting the generator to the external power supply.
• The parts of the device that may come into contact with the skin do not normally cause any allergic reaction.
Although the coil's covering material is hypoallergenic and biocompatible, it is recommended not to place
the coil in direct contact with the skin, but to place it on a light garment, especially if there is any reddening
or irritation in the area of application.
• Clean the coil regularly, taking care to disconnect the coil from the generator using neutral detergents.
• Any wear on the coil coating due to use does not affect the effectiveness of the treatment. However, in the
event of a loss of some of the coating, the coil must be replaced.
• When using the coil under heavy blankets, the coil surface may become overheated. If this is the case,
perform the treatment without covering the coil.
• In any case, the generator must not be covered to allow ventilation whilst it is in operation.
• Using the device in environments with a temperature above 30°C, although possible, could cause the coil
surface to slightly overheat and this may be uncomfortable for the user.
• When charging or operating via the mains, it is normal for the battery to get hot; for this reason, the
generator should not be placed in direct contact with the body when charging or operating via the mains.
• The elastic band can be washed just like any other garment.
11

ENGLISH
IGEA I-ONE - User Manual
Page 11
5.8 Cleaning the device
The device must be used in accordance with normal hygiene standards and must be cleaned regularly. The presence
near to the device of hair or dust, as well as exposure to direct sunlight, while not causing it to malfunction, should
be avoided.
Before cleaning the generator, make sure it is switched off and disconnected from the power supply: the generator
can be cleaned with a cloth that has been slightly dampened with neutral detergents. Do not use solvents or
aggressive cleaning agents.
Clean the coil regularly using neutral detergents, taking care to disconnect the coil from the generator.
6. PROBLEM SOLVING
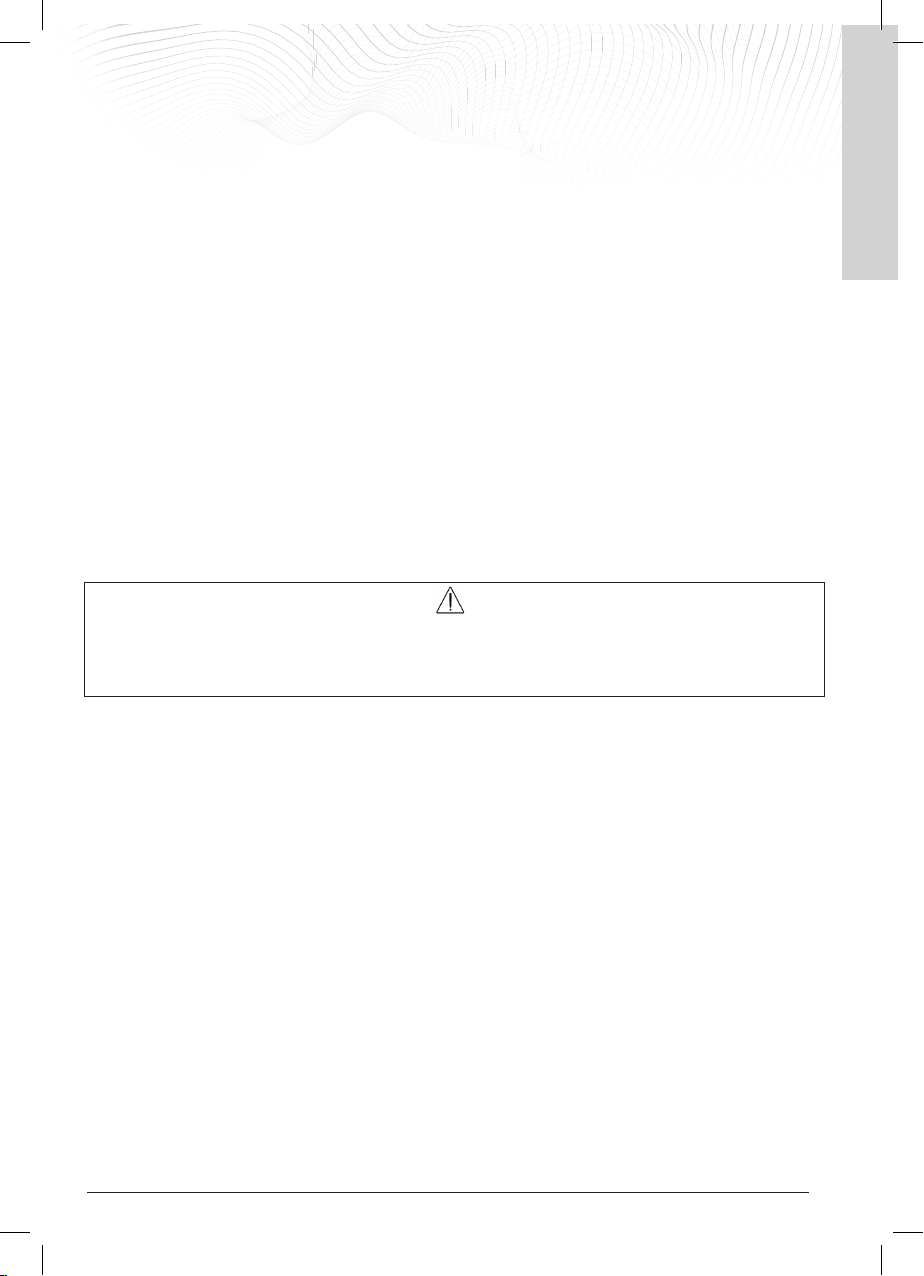
6.1 Error messages
The device recognises and reports any malfunction statuses. Below are the reports it provides and the actions to be
taken to restore its operation.
Display
LED and alarms
Problem and solution
The LED lights up RED.
The generator vibrates.
There is a rapid sequence of 3 beeps every
3 seconds.
In the centre of the display, the image of
the coil appears with the warning triangle
and the suggestion of what to do to
resolve the anomaly.
'Coil Failure’ warning
The coil is connected to the generator but it
has no power.
Switch off the generator, try removing and
reinserting the coil, and switch it on again.
If, when switching it back on, the device
signals the anomaly again, switch the
generator off again and contact IGEA
Customer Service for a replacement coil.
The LED lights up YELLOW.
The generator vibrates.
There is a rapid sequence of 3 beeps every
3 seconds.
In the centre of the display, the image of
the coil appears with a question mark and
the suggestion of what to do to resolve
the anomaly.
'Coil Absent’ warning
The device was switched on and the PLAY
button was pressed, but the coil is not
connected to the generator.
The user must connect the coil to the
generator in order to resolve the anomaly
and start/resume treatment by pressing the
PLAY button on the screen.
NOTE: If the coil is not connected to the
generator, the device switches off
automatically after 30 seconds
The LED at the top flashes with an
alternating green and red light
The device beeps 3 times every 5 seconds
The image on the display informs the
patient that the device requires
maintenance and invites them to contact
IGEA Customer Service
After 1 minute of inactivity, the I-ONE
shuts down.
The I-ONE device has a verification system to
ensure it works properly.
When this message is displayed, the system
detects the need for a standard maintenance
check.
This check must be agreed upon with the
IGEA Customer Service.
IGEA I-ONE - User Manual
Page 12
6.2 Anomalies or blocked device
6.2.1 The device will not switch on and will not charge.
External interference or the battery being completely flat (e.g. after prolonged non-use) can block the device,
stopping it from working as normal.
To unlock it, proceed as follows:
1. Connect the external power supply to the generator and wait for up to 30 seconds; the battery should begin
charging as described in section 3.1
2. If charging has not started after 30 seconds, leave the power supply connected to the generator, press and
hold the ON/OFF button for at least 8 seconds.
Continuously pressing the ON/OFF button for 8 seconds causes a generator RESET.
When the button is released, the battery should begin charging.
Leave the generator on charge until it is fully charged before using the device.
If even after the device has been RESET the battery does not start to charge, please contact IGEA Customer Service.
6.2.2 Blocked device during normal operation.
External interference from other electrical and electronic devices in the area of use (modems, mobile phones,
cordless devices, etc.) may interfere with the device and cause it to get blocked.
Should the device become blocked and not respond to normal commands, perform a RESET as described in the
previous paragraph.
6.2.3 Technical Support
In the event of a permanent failure, contact technical support to repair the device.
Technical support for the device is the sole responsibility of the manufacturer IGEA S.p.A. In the
event of a fault or in any case in which the device needs to be serviced, the user must contact the
IGEA S.p.A. support centre.
Tel. 059 699 600 - Fax. 059 695 778 e-mail: info@igeamedical.com
7. SAFETY INSTRUCTIONS
7.1 Warnings and Recommendations
For the device to work safely and at its best, the following recommendations must be strictly followed:
• Read this manual before starting to use the device.
• The I-ONE must be used by people who are capable of independently understanding and implementing the
instructions provided in this manual; otherwise, and if it is being used on children, I-ONE may only be used
under the supervision of people who are capable of understanding and implementing the instructions
provided in this manual.
• When the generator is connected to the external power supply, position the device so that the power
connector can be easily removed if necessary.
• Keep the device away from children and pets, if any.
• Caution: connecting cables may cause a strangulation hazard if they are not used correctly.
• Do not use the device in the presence of flammable gases.
• Do not connect any part of the device to other equipment or devices.
• Do not connect any parts to the I-ONE which are not intended for use and not supplied by the manufacturer.
• Do not handle any parts of the device with wet hands, and more specifically, do not connect the external
power supply to the mains.
• Do not immerse any of the constituent parts of the device in water or liquids of any kind and do not pour
liquids on them; in the event that the generator accidentally comes into contact with liquids, do not use
the device and return it to the support centre or the manufacturer for inspection/repair.
• It is recommended not to cover the generator during treatment delivery or charging so as to ensure
ventilation.
12
ENGLISH
IGEA I-ONE - User Manual
Page 12
6.2 Anomalies or blocked device
6.2.1 The device will not switch on and will not charge.
External interference or the battery being completely flat (e.g. after prolonged non-use) can block the device,
stopping it from working as normal.
To unlock it, proceed as follows:
1. Connect the external power supply to the generator and wait for up to 30 seconds; the battery should begin
charging as described in section 3.1
2. If charging has not started after 30 seconds, leave the power supply connected to the generator, press and
hold the ON/OFF button for at least 8 seconds.
Continuously pressing the ON/OFF button for 8 seconds causes a generator RESET.
When the button is released, the battery should begin charging.
Leave the generator on charge until it is fully charged before using the device.
If even after the device has been RESET the battery does not start to charge, please contact IGEA Customer Service.
6.2.2 Blocked device during normal operation.
External interference from other electrical and electronic devices in the area of use (modems, mobile phones,
cordless devices, etc.) may interfere with the device and cause it to get blocked.
Should the device become blocked and not respond to normal commands, perform a RESET as described in the
previous paragraph.
6.2.3 Technical Support
In the event of a permanent failure, contact technical support to repair the device.
Technical support for the device is the sole responsibility of the manufacturer IGEA S.p.A. In the
event of a fault or in any case in which the device needs to be serviced, the user must contact the
IGEA S.p.A. support centre.
Tel. 059 699 600 - Fax. 059 695 778 e-mail: info@igeamedical.com
7. SAFETY INSTRUCTIONS
7.1 Warnings and Recommendations
For the device to work safely and at its best, the following recommendations must be strictly followed:
• Read this manual before starting to use the device.
• The I-ONE must be used by people who are capable of independently understanding and implementing the
instructions provided in this manual; otherwise, and if it is being used on children, I-ONE may only be used
under the supervision of people who are capable of understanding and implementing the instructions
provided in this manual.
• When the generator is connected to the external power supply, position the device so that the power
connector can be easily removed if necessary.
• Keep the device away from children and pets, if any.
• Caution: connecting cables may cause a strangulation hazard if they are not used correctly.
• Do not use the device in the presence of flammable gases.
• Do not connect any part of the device to other equipment or devices.
• Do not connect any parts to the I-ONE which are not intended for use and not supplied by the manufacturer.
• Do not handle any parts of the device with wet hands, and more specifically, do not connect the external
power supply to the mains.
• Do not immerse any of the constituent parts of the device in water or liquids of any kind and do not pour
liquids on them; in the event that the generator accidentally comes into contact with liquids, do not use
the device and return it to the support centre or the manufacturer for inspection/repair.
• It is recommended not to cover the generator during treatment delivery or charging so as to ensure
ventilation.
13

ENGLISH
IGEA I-ONE - User Manual
Page 13
• When using the coil under heavy blankets, the coil surface may become overheated: if the temperature of the coil
causes discomfort, it is advisable to carry out the therapy without covering the coil.
• In environments where the temperature is above 30°C you may experience warming of the coil surface; if the
temperature of the coil causes discomfort, it is advisable to split the daily therapy time into several sessions lasting
no less than two hours each.
• Keep the generator away from the body when charging the battery.
• During use, the display may exceed a temperature of 41°C, but this is in any case below the regulatory limit given
the limited patient contact time.
• Clean the coil regularly, using only neutral detergents; do not use solvents or aggressive cleaning agents. Cleaning
must be carried out when the coil is disconnected from the generator. The coil is for individual patient use only.
• The generator can be cleaned using a cloth that has been slightly dampened with water or a neutral detergent; the
generator must be cleaned when the unit is switched off.
• Avoid any mechanical shocks to the device during transportation or movement.
• In the event of a collision or fall that causes the device to break and/or open, the device and all its parts must be
collected and placed in the transport container and not used for any purpose. If the device is connected to the
mains socket, first remove the power supply unit from the mains socket. The user should then contact the
manufacturer to return the device and to possibly repair it.
• Before each treatment session, check the integrity of the connection cable between the generator and the coil; if
it is damaged, replace the coil with a new, undamaged one supplied by the manufacturer.
• Before using the external power supply, check that the casing and cable are not damaged; if they are, replace the
power supply with one supplied by the manufacturer.
• Do not expose the device to heat sources and do not throw it into fire as there is a danger of explosion!
• The battery is a polluting waste that must be disposed of according to current disposal regulations.
• If the device is left unused for long periods of time, the battery may go completely flat and must be fully charged
before starting treatment again.
• Caution: only use the power supply unit supplied to charge the battery. The use of other devices could cause
damage to the generator, battery or user for which the manufacturer waives any liability.
• The device is equipped with self-monitoring mechanisms to ensure it is working correctly; any anomalies are
signalled by the device and are described in the instruction manual.
• Any serious accident occurring during the use of the medical device and related to it must be reported by the user
to the manufacturer, who will notify the competent authority of the member state where the user and/or patient
is established.
• The device can be used with implantable medical devices (e.g. joint prostheses) with the CE conformity certification.
There are no restrictions on the use of this combination since clinical studies with similar devices indicate that
stimulation relieves pain in subjects with mobilised and painful prostheses and no contraindications have emerged.
7.2 Maintenance
The device is assembled by the manufacturer and requires a specific mechanical tool to open it. This is so as to
prevent tampering and/or unauthorised repair attempts by the user or third parties.
Any work on the device that requires the generator to be opened must be carried out by the manufacturer or
authorised technical support; otherwise the safety of the device is no longer guaranteed.
• In order to ensure a reliable performance, the manufacturer recommends that the device undergoes a
routine maintenance procedure and checks on the operating parameters at intervals no greater than 24
months. This maintenance should be requested from the IGEA Customer Support.
• The power supply battery contained in the device cannot be removed/replaced by the user. If necessary, the
battery may only be replaced by the manufacturer or by the manufacturer's authorised technical support.
• The manufacturer recommends repeating the safe tests on the device to check that safety standards are
being continuously maintained, at intervals no greater than 24 months. By agreement with the customer,
IGEA can provide the recommended electrical safety inspection service.
7.3 Contraindications and side effects
There are no known contraindications to the use of I-ONE and no side effects attributable to the treatment have
been observed. However, the following precautions must be taken:
IGEA I-ONE - User Manual
Page 14
• In the case of an established or presumed pregnancy, although no adverse effects attributable to the treatment
have been described, treatment in the pelvic area should be avoided as a precautionary measure. In any case,
always inform the doctor who prescribed the treatment, who will assess the need for continuation/interruption
on a case-by-case basis.
• Pacemaker recipients may receive treatment with the I-ONE, but they must notify the prescribing doctor, who
will assess whether to start/continue the treatment.
• Fewer than 2 in every 1000 patients report heat/burning during treatment. In this case, it is recommended that
in the first week of treatment the daily sessions be divided up into several lasting one hour each; the treatment
should then be gradually increased until the standard regimen is reached. The burning sensation disappears when
the treatment is interrupted.
• While not presenting the use of the device, usually, any contraindications in conjunction with the use of
medication, notify the doctor prescribing the therapy of any medications you take.
• There are no restrictions on the use of the device with simultaneous implantable medical devices (e.g. joint
prostheses), but these must be CE-marked and are not supplied by the manufacturer. Clinical studies with similar
devices indicate that stimulation relieves pain in subjects with mobilised and painful prostheses and no
contraindications have emerged.
7.4 Electromagnetic Compatibility
The I-ONE has been tested and certified as compliant with medical device electromagnetic compatibility standards
and declared suitable for use at home.
The I-ONE can be used in conjunction with other electrical or electronic devices if they too conform to current
standards without causing interference or receiving interference. However, the following general requirements must
be observed:
• The I-ONE must not be used adjacent to or on top of other devices. If the I-ONE must be used in this way, the
medical device must be observed to ensure it works correctly in the configuration in which it is used;
• The I-ONE must be positioned and used in accordance with the EMC information provided later in this manual.
• The I-ONE must not be used at the same time as other treatments or applications of electromedical device that
involve the release of energy to the patient's body, particularly if they use high-frequency signals, as these
signals could interfere with the operation of the I-ONE and cause undesirable alterations in the treatment signal.
• The use of accessories and cables other than those specified and supplied directly by the I-ONE manufacturer
may result in increased emissions or lower immunity for the I-ONE and cause it not to work properly.
• Portable and mobile RF communication devices, including peripherals such as antenna cables and external
antennas, should be kept more than 30 cm away from all I-ONE components including cables. Failure to do so
could lead to a deterioration in the performance of the medical device.
Blocked device: Electromagnetic interference, in particular electrostatic discharges of a power greater than
8kV, could alter the normal operation of the I-ONE and cause the device to become blocked.
In the event of a blocked device, or in any case in which the device does not switch off or does not react when
the On/Off button is pressed, reset the device to restore normal operation following the instructions in section
6.2.
7.5 Biological safety
The safety of treatment with the I-ONE has been extensively verified; all tests showed the absence of adverse
treatment effects.
8. MANUFACTURER’S LIABILITY
The manufacturer is only responsible for the safety, reliability and performance of the I-ONE if:
• The device is used in accordance with the operating instructions described in this manual.
• The device is not opened or tampered with in any way by the user or other unauthorised persons.
• Only the power supply unit supplied directly by the manufacturer as a spare should be used.
• The external power supply is used exclusively for the I-ONE device as described in this manual.
• Regular inspections, modifications and/or repairs are carried out by personnel authorised by IGEA.
14

ENGLISH
IGEA I-ONE - User Manual
Page 13
• When using the coil under heavy blankets, the coil surface may become overheated: if the temperature of the coil
causes discomfort, it is advisable to carry out the therapy without covering the coil.
• In environments where the temperature is above 30°C you may experience warming of the coil surface; if the
temperature of the coil causes discomfort, it is advisable to split the daily therapy time into several sessions lasting
no less than two hours each.
• Keep the generator away from the body when charging the battery.
• During use, the display may exceed a temperature of 41°C, but this is in any case below the regulatory limit given
the limited patient contact time.
• Clean the coil regularly, using only neutral detergents; do not use solvents or aggressive cleaning agents. Cleaning
must be carried out when the coil is disconnected from the generator. The coil is for individual patient use only.
• The generator can be cleaned using a cloth that has been slightly dampened with water or a neutral detergent; the
generator must be cleaned when the unit is switched off.
• Avoid any mechanical shocks to the device during transportation or movement.
• In the event of a collision or fall that causes the device to break and/or open, the device and all its parts must be
collected and placed in the transport container and not used for any purpose. If the device is connected to the
mains socket, first remove the power supply unit from the mains socket. The user should then contact the
manufacturer to return the device and to possibly repair it.
• Before each treatment session, check the integrity of the connection cable between the generator and the coil; if
it is damaged, replace the coil with a new, undamaged one supplied by the manufacturer.
• Before using the external power supply, check that the casing and cable are not damaged; if they are, replace the
power supply with one supplied by the manufacturer.
• Do not expose the device to heat sources and do not throw it into fire as there is a danger of explosion!
• The battery is a polluting waste that must be disposed of according to current disposal regulations.
• If the device is left unused for long periods of time, the battery may go completely flat and must be fully charged
before starting treatment again.
• Caution: only use the power supply unit supplied to charge the battery. The use of other devices could cause
damage to the generator, battery or user for which the manufacturer waives any liability.
• The device is equipped with self-monitoring mechanisms to ensure it is working correctly; any anomalies are
signalled by the device and are described in the instruction manual.
• Any serious accident occurring during the use of the medical device and related to it must be reported by the user
to the manufacturer, who will notify the competent authority of the member state where the user and/or patient
is established.
• The device can be used with implantable medical devices (e.g. joint prostheses) with the CE conformity certification.
There are no restrictions on the use of this combination since clinical studies with similar devices indicate that
stimulation relieves pain in subjects with mobilised and painful prostheses and no contraindications have emerged.
7.2 Maintenance
The device is assembled by the manufacturer and requires a specific mechanical tool to open it. This is so as to
prevent tampering and/or unauthorised repair attempts by the user or third parties.
Any work on the device that requires the generator to be opened must be carried out by the manufacturer or
authorised technical support; otherwise the safety of the device is no longer guaranteed.
• In order to ensure a reliable performance, the manufacturer recommends that the device undergoes a
routine maintenance procedure and checks on the operating parameters at intervals no greater than 24
months. This maintenance should be requested from the IGEA Customer Support.
• The power supply battery contained in the device cannot be removed/replaced by the user. If necessary, the
battery may only be replaced by the manufacturer or by the manufacturer's authorised technical support.
• The manufacturer recommends repeating the safe tests on the device to check that safety standards are
being continuously maintained, at intervals no greater than 24 months. By agreement with the customer,
IGEA can provide the recommended electrical safety inspection service.
7.3 Contraindications and side effects
There are no known contraindications to the use of I-ONE and no side effects attributable to the treatment have
been observed. However, the following precautions must be taken:
IGEA I-ONE - User Manual
Page 14
• In the case of an established or presumed pregnancy, although no adverse effects attributable to the treatment
have been described, treatment in the pelvic area should be avoided as a precautionary measure. In any case,
always inform the doctor who prescribed the treatment, who will assess the need for continuation/interruption
on a case-by-case basis.
• Pacemaker recipients may receive treatment with the I-ONE, but they must notify the prescribing doctor, who
will assess whether to start/continue the treatment.
• Fewer than 2 in every 1000 patients report heat/burning during treatment. In this case, it is recommended that
in the first week of treatment the daily sessions be divided up into several lasting one hour each; the treatment
should then be gradually increased until the standard regimen is reached. The burning sensation disappears when
the treatment is interrupted.
• While not presenting the use of the device, usually, any contraindications in conjunction with the use of
medication, notify the doctor prescribing the therapy of any medications you take.
• There are no restrictions on the use of the device with simultaneous implantable medical devices (e.g. joint
prostheses), but these must be CE-marked and are not supplied by the manufacturer. Clinical studies with similar
devices indicate that stimulation relieves pain in subjects with mobilised and painful prostheses and no
contraindications have emerged.
7.4 Electromagnetic Compatibility
The I-ONE has been tested and certified as compliant with medical device electromagnetic compatibility standards
and declared suitable for use at home.
The I-ONE can be used in conjunction with other electrical or electronic devices if they too conform to current
standards without causing interference or receiving interference. However, the following general requirements must
be observed:
• The I-ONE must not be used adjacent to or on top of other devices. If the I-ONE must be used in this way, the
medical device must be observed to ensure it works correctly in the configuration in which it is used;
• The I-ONE must be positioned and used in accordance with the EMC information provided later in this manual.
• The I-ONE must not be used at the same time as other treatments or applications of electromedical device that
involve the release of energy to the patient's body, particularly if they use high-frequency signals, as these
signals could interfere with the operation of the I-ONE and cause undesirable alterations in the treatment signal.
• The use of accessories and cables other than those specified and supplied directly by the I-ONE manufacturer
may result in increased emissions or lower immunity for the I-ONE and cause it not to work properly.
• Portable and mobile RF communication devices, including peripherals such as antenna cables and external
antennas, should be kept more than 30 cm away from all I-ONE components including cables. Failure to do so
could lead to a deterioration in the performance of the medical device.
Blocked device: Electromagnetic interference, in particular electrostatic discharges of a power greater than
8kV, could alter the normal operation of the I-ONE and cause the device to become blocked.
In the event of a blocked device, or in any case in which the device does not switch off or does not react when
the On/Off button is pressed, reset the device to restore normal operation following the instructions in section
6.2.
7.5 Biological safety
The safety of treatment with the I-ONE has been extensively verified; all tests showed the absence of adverse
treatment effects.
8. MANUFACTURER’S LIABILITY
The manufacturer is only responsible for the safety, reliability and performance of the I-ONE if:
• The device is used in accordance with the operating instructions described in this manual.
• The device is not opened or tampered with in any way by the user or other unauthorised persons.
• Only the power supply unit supplied directly by the manufacturer as a spare should be used.
• The external power supply is used exclusively for the I-ONE device as described in this manual.
• Regular inspections, modifications and/or repairs are carried out by personnel authorised by IGEA.
15

ENGLISH
IGEA I-ONE - User Manual
Page 15
• The device is subjected to a function parameter check and safe-test at least every 24 months.
Please contact the manufacturer for further information or updates.
Manufacturer:
IGEA S.p.A. Via Parmenide 10/A, 41012 Carpi (MO) ITALY
9. DEVICE RETURNS
If the device is to be returned to IGEA, the user is requested to use the original packaging complete with all its parts.
The adjacent image shows the correct positioning of the various components to ensure that they are adequately
protected.
Place the external power supply in the rectangular
hole on the right with the plug pointing to the left,
passing the cable through the space below the hole.
Insert the elastic band supporting the coil into the
housing next to the external power supply.
Then insert the I-ONE CBA04 generator into the hole
on the left, pressing slightly to fit it into the housing.
Insert the coil into the dedicated central space.
10.TECHNICAL DATA
The I-ONE complies with EU Medical Device Regulation
2017/745 and is marked
0051
under the control of IMQ.
The I-ONE has an expected lifetime of 5 years after being placed on the market.
The applied parts (coils) have an expected lifetime of 12 months after being placed on the market.
I-ONE Generator - CBA04
Power supply voltage : 11.1 VDC
Maximum current consumption : 0.250 A
Maximum input power : 3 W
Classification according to EN 60601-1 : Class II device - Type BF
Classification according to MDR 2017/745 EU : Class IIa device
Li-ion 11.1 VDC/1100 mAh
or Li-ion 11.1 VDC/1350 mAh rechargeable battery
Do not expose the battery to heat sources and do not throw it into fire as there is a danger of explosion!
Do not immerse the battery pack in liquids or pour liquids onto it.
The battery is a polluting waste that must be disposed of according to current disposal regulations.
External power supply
Model
ME30A1541B01
Brand
SL Power
Input voltage
230 VAC (100-240)
Network frequency
50-60 Hz
Max. current input
0.150 A
Output voltage
15 VDC
Max. output current
2.0 A
Short-circuit protection
Continued
Insulation class
II
The power supply model supplied by the manufacturer is approved according to EN60601-1 and EN60601-1-2.
Exclusively use the power supply unit supplied by the manufacturer.
IGEA I-ONE - User Manual
Page 16
Coil drive signal characteristics and magnetic field strength:
Signal type : Rectangular signal
Frequency : 75 Hz ± 5%
Pulse width : 1.0 ± 0.1 milliseconds
Magnetic field intensity produced : 10-18 Gauss (peak value)
Method of use:
Device with internal rechargeable electrical source with specified power supply unit. Device for
continuous operation not to be used in the presence of flammable anaesthetic mixtures with air, oxygen or nitrous
oxide.
Device with IP22 protection class. The IP22 class offers protection against the entrance of solids with a diameter
> 12 mm and protection against the entrance of water or rain drops falling at an angle ≤ 15° from the vertical of the
device.
Conditions of use of the device:
Room temperature : 5 - 34 °C
Relative humidity : 15% - 90% (free from condensation)
Atmospheric pressure : 700-1060hPa
Transportation and storage conditions:
Room temperature : -25 - +70°C
Relative humidity : 0 % at -25°C to 90% (non-condensing) at 70°C
Atmospheric pressure : 500 - 1060hPa
Storage conditions between uses:
Between one session and the next, the device must be stored in its packaging or in another clean and dry place under
the same environmental conditions as when it was intended for use.
Warning! The device must not be used in places where there is a danger of explosion.
Restoration of environmental conditions of use
If the device comes from a place with a different temperature (e.g. due to transportation or storage), wait about
10 minutes for it to adjust to the room temperature before using it.
End-of-life disposal
The I-ONE device and each of its parts cannot be disposed of as municipal waste but are subject to a separate
collection according to the procedures established by the local authorities.
10.1 Table 1 - Electromagnetic Emissions
MANUFACTURER'S GUIDANCE AND DECLARATION - ELECTROMAGNETIC EMISSIONS
The I-ONE - CBA04 model is usable in the specified electromagnetic environment. The user must ensure that it is
used in an electromagnetic environment with the characteristics described below.
Emission Test
Compliance
Electromagnetic Environment
RF emissions - CISPR 11
Group 1
The I-ONE - CBA04 model generates radio frequency signals solely
as a consequence of the internal electronic circuits. Its radio
emissions are very low and are unlikely to cause radio interference
with nearby devices.
RF emissions - CISPR 11
Class B
The I-ONE - CBA04 model is suitable for use in any environment,
including households and those directly connected to a low-voltage
public mains supply that supplies buildings used for domestic
purposes.
Harmonic Emissions
EN 61000-3-2
Class A
Voltage fluctuation / flicker emissions
EN 61000-3-3
Compliant
10.2 Table 2 - Electromagnetic Immunity
Manufacturer's guidance and declaration - electromagnetic immunity
Proof of Immunity
Test Level
EN 60601-1-2
Compliance
Level
Electromagnetic Environment
16

ENGLISH
IGEA I-ONE - User Manual
Page 16
Coil drive signal characteristics and magnetic field strength:
Signal type : Rectangular signal
Frequency : 75 Hz ± 5%
Pulse width : 1.0 ± 0.1 milliseconds
Magnetic field intensity produced : 10-18 Gauss (peak value)
Method of use:
Device with internal rechargeable electrical source with specified power supply unit. Device for
continuous operation not to be used in the presence of flammable anaesthetic mixtures with air, oxygen or nitrous
oxide.
Device with IP22 protection class. The IP22 class offers protection against the entrance of solids with a diameter
> 12 mm and protection against the entrance of water or rain drops falling at an angle ≤ 15° from the vertical of the
device.
Conditions of use of the device:
Room temperature : 5 - 34 °C
Relative humidity : 15% - 90% (free from condensation)
Atmospheric pressure : 700-1060hPa
Transportation and storage conditions:
Room temperature : -25 - +70°C
Relative humidity : 0 % at -25°C to 90% (non-condensing) at 70°C
Atmospheric pressure : 500 - 1060hPa
Storage conditions between uses:
Between one session and the next, the device must be stored in its packaging or in another clean and dry place under
the same environmental conditions as when it was intended for use.
Warning! The device must not be used in places where there is a danger of explosion.
Restoration of environmental conditions of use
If the device comes from a place with a different temperature (e.g. due to transportation or storage), wait about
10 minutes for it to adjust to the room temperature before using it.
End-of-life disposal
The I-ONE device and each of its parts cannot be disposed of as municipal waste but are subject to a separate
collection according to the procedures established by the local authorities.
10.1 Table 1 - Electromagnetic Emissions
MANUFACTURER'S GUIDANCE AND DECLARATION - ELECTROMAGNETIC EMISSIONS
The I-ONE - CBA04 model is usable in the specified electromagnetic environment. The user must ensure that it is
used in an electromagnetic environment with the characteristics described below.
Emission Test
Compliance
Electromagnetic Environment
RF emissions - CISPR 11
Group 1
The I-ONE - CBA04 model generates radio frequency signals solely
as a consequence of the internal electronic circuits. Its radio
emissions are very low and are unlikely to cause radio interference
with nearby devices.
RF emissions - CISPR 11
Class B
The I-ONE - CBA04 model is suitable for use in any environment,
including households and those directly connected to a low-voltage
public mains supply that supplies buildings used for domestic
purposes.
Harmonic Emissions
EN 61000-3-2
Class A
Voltage fluctuation / flicker emissions
EN 61000-3-3
Compliant
10.2 Table 2 - Electromagnetic Immunity
Manufacturer's guidance and declaration - electromagnetic immunity
Proof of Immunity
Test Level
EN 60601-1-2
Compliance
Level
Electromagnetic Environment
17

ENGLISH
IGEA I-ONE - User Manual
Page 17
Electrostatic Discharge (ESD)
IEC 61000-4-2
± 8 kV contact
± 15 kV air
Test Level
IEC 60601-1-2
Any environment including at home
Irradiated RF
IEC 61000-4-3
10 V/m
80 MHz to 2.7 GHz
Test Level
IEC 60601-1-2
Any environment including at home with portable
and mobile RF devices kept as far away from the I-
ONE - CBA04 model as possible, including
connecting cables.
Minimum distance 30 cm
Fast electrical
transients/bursts
IEC 61000-4-4
± 2 kV per supply line
± 1 kV per input/output
line
Test Level
IEC 60601-1-2
Any environment including at home
Surges
IEC 61000-4-5
± 1 kV between phases
± 2 kV between phase
and earth
Test Level
IEC 60601-1-2
Any environment including at home
Conducted RF
IEC 61000-4-6
3 V eff.
150 kHz to 80 MHz
6 V - ISM frequencies
and amateur radio
band
Test Level
IEC 60601-1-2
Any environment including at home with portable
and mobile RF devices kept as far away from the I-
ONE - CBA04 model as possible, including
connecting cables.
Minimum distance 30 cm
Voltage dips, short
interruptions and voltage
variations on power supply
input lines
IEC 61000-4-11
10 ms - 0% at 0°, 45°,
90°, 135°, 180°. 225°,
270°, 315°
20 ms - 0% at 0°
500 ms - 70% at 0°
5 s - 0%
Test Level
IEC 60601-1-2
Any environment including at home
Magnetic field at mains
frequency (50/60 Hz)
IEC 61000-4-8
30 A/m
Test Level
IEC 60601-1-2
Any environment including at home
10.3 Immunity to proximity fields from RF wireless communication devices
Portable or mobile RF communication devices that may be present in the home such as wireless telephones,
mobile phones, devices for wireless connection to the internet and similar, must be kept away from the I-ONE
device to avoid the risk of interference. The recommended minimum separation distance depends on the output
power of the RF device and the transmission frequency. The user can help prevent electromagnetic interference
by maintaining a minimum distance between portable and mobile RF communication systems and the I-ONE using
the table below as a reference.
Test
Frequency
(MHz)
Band
(MHz)
Type of Service
Modulation
Maximum
Power
(W)
Distance
(m)
Test immunity
level
(V/m)
385
380 -390
TETRA 400
Pulse
modulation
18 Hz
1.8
0.3
27
450
430 - 470
GMRS 460, FRS 460
FM
± 5 kHz deviation
1 kHz sine
2
0.3
28
710
704 - 787
LTE Band 13,
17
Pulse
modulation
217 Hz
0.2
0.3
9
745
780
810
800 - 960
GSM 800/900,
TETRA 800, iDEN 820,
CDMA 850,
LTE Band 5
Pulse
modulation
18 Hz
2
0.3
28
870
930
1 720
1700 -1990
Pulse
2
0.3
28
IGEA I-ONE - User Manual
Page 18
1 845
GSM 1800; CDMA
1900; GSM 1900;
DECT;
LTE Band 1, 3,
4, 25; UMTS
modulation
217 Hz
1 970
2 450
2400 -2570
Bluetooth, W LAN,
802.11 b/g/n,
RFID 2450,
LTE Band 7
Pulse
modulation
217 Hz
2
0.3
28
5 240
5100 -5800
W LAN 802.11 a/n
Pulse
modulation
217 Hz
0.2
0.3
9
5 500
5 785
The immunity levels given in the table are met as long as the device is kept at a distance of at least 30 cm from any
possible source of RF interference.
The I-ONE - CBA04 complies with all test levels with a distance >= 30 cm.
18
This manual suits for next models
1
Table of contents
Languages:
Other IGEA Medical Equipment manuals
Popular Medical Equipment manuals by other brands

ARJO HUNTLEIGH
ARJO HUNTLEIGH Essence 2000 Instructions for use

Contec
Contec CMS-50D Plus instructions

Akces-Med
Akces-Med PARAMOBIL manual
Cooper Surgical
Cooper Surgical ALLY Uterine Positioning System Instructions for use

Care Fusion
Care Fusion MicroLab operating manual

HOMEAIDE
HOMEAIDE Easy Air Mini NI-50 instructions
B. Braun
B. Braun Aesculap Neurosurgery Instructions for use/Technical description

Hillrom
Hillrom Trumpf Medical iLED 7 instruction manual

B. Braun
B. Braun Aesculap Detachable Rongeurs Disassembly

DRTECH
DRTECH EVS4343W user manual
C-me
C-me VET-PRO VIP 2000 Operator's manual

Pro-tec
Pro-tec UCARE user manual