Trends Audio Science mad Crystal Growing Lab User manual

14 Safe, Educational Experiments
Instruction Manual
WARNING!
Not suitable for children under 10 years. For use under adult supervision. Contains some chemicals which present a hazard to health. Read the instructions before use, follow
them and keep them for reference. Do not allow chemicals to come into contact with any part of the body, particularly the mouth and eyes. Keep small children and animals
away from experiments. Keep the experimental set out of reach of children under 10 years old.
Imported by Trends UK Ltd,
Trends UK Ltd,
Greatworth Hall,
Banbury,
OX17 2DH, UK
England,
UK
Email: [email protected]
Item No. SM21
www.trendsuk.co.uk
Customer Services:
+4 4 (0)1702 20 8175
Please retain the information in this manual
for future reference.
Colour, designs and decorations may vary
from those shown in the photographs.
Printed in China.
AGES 10+

Includes:
• Monoammonium phosphate
• Aluminium potassium sulphate
• 3 petri dishes
• 5 plastic containers with lids
• 2 plastic moulds
• 1 large measuring cup with lid
• 1 small measuring cup with lid
• 2 measuring spoons
Advice For Supervising Adults 2
First Aid Advice 3
Safety Rules 3
Introduction To Crystal Growing 4
Crystal Growing Activities 5-11
Notes 12
Water Molecule Models 13
Orange citrine 40g
Pink quartz 40g
Emerald green 40g
Marine blue 40g
Lilac geode 40g
Crystal Growing Chemicals:
Frosty diamond 150g
Rama quartz 150g
Red ruby geode 110g
Pink frost 110g
Yellow geode 110g
Moss geode 110g
• Read and follow the instructions, the safety rules and the first aid
information, and keep them for future reference.
• The incorrect use of chemicals can cause injury and damage to health.
Only carry out those experiments that are listed in the instructions.
• This crystal growing kit is for use only by children over 10 years of age.
• Because children’s abilities vary so much, even within age groups,
supervising adults should exercise discretion as to which preparations are
suitable and safe for them. The instructions should enable supervisors to
assess any experiment to establish its suitability for a particular child.
• The supervising adult should discuss the warnings and safety information
with the children before commencing the experiments. Particular attention
should be paid to the safe handling of the materials in the bottles and all
chemical preparations made in the activities.
• The area surrounding the experiment should be kept clear of any
obstructions and away from the storage of food. It should be well lit,
ventilated and close to a water supply. A solid table with a heat resistant
top should be provided.
• A separate tin or bucket should be used for the disposal of solid waste
materials. Any wasted solution should be poured down a drain but never
into a sink.
• Crystal growing is a slow process and requires patience to get good
results. TO AVOID DISAPPOINTMENT, PLEASE NOTE: You should wait
a minimum of 12 hours for crystals to grow, but better results can be
obtained by waiting for up to a week.
First Aid Advice – Chemicals
Doctor:
Hospital:
Chemicals supplied in this kit:
• Monoammonium phosphate mixed with food colouring
• Aluminium potassium sulphate mixed with food colouring
In case of eye contact: Wash out eye with plenty of water, holding eye open if
necessary. Seek immediate medical advice.
In case of skin contact and burns: Wash affected area with plenty of cold water
for at least 10 minutes.
If swallowed: Wash out mouth with water, drink some fresh water. Do not induce
vomiting. Seek immediate medical advice.
In case of inhalation: Remove person to fresh air.
In case of skin contact and burns: Wash affected area with plenty of cold water
for at least 10 minutes.
In case of doubt seek medical advice without delay. Take the chemical together
with the container with you.
In case of injury always seek medical advice.
NOTE: First aid information may also be found in the instructions for carrying out
the experiment.
Adults – please complete this section before using the kit. • Wash with warm soapy water, rinse and dry with a soft cloth.
• These goggles are only to be used with the contents and instructions supplied.
If goggles become damaged, do not attempt to repair; discard immediately.
• Materials which may come into contact with the wearer’s skin could cause allergic
reactions to susceptible individuals.
Safety Goggles
Safety Goggles continued...
Safety Rules
Instructions for use, storage and maintenance
• Hold goggles with one hand, if possible without touching the lens. Pull the elastic head
band over the back of your head, just above the ears so that the goggles sit on your
forehead. Carefully pull the goggles down over the eyes and adjust the strap for a snug
and comfortable fit. Ensure the goggles are kept clean and dry, and cannot come into
contact with loose chemicals or sharp objects.
• Read and follow these instructions before use, follow them and keep them for reference.
• Keep young children and animals away from the experimental area.
• Store this experimental set and final crystals out of reach of children under 10 years of age.
• Clean all equipment after use.
• Wash hands after carrying out experiments.
• Do not eat or drink in the experimental area.
• Do not allow chemicals to come into contact with the eyes or mouth.
• Do not use any equipment which has not been supplied with the set or recommended
in the instructions for use.
• Make sure that all containers are fully closed and properly stored after use.
• Ensure that all empty containers and non-reclosable packaging are disposed of properly.
• Do not apply any substances or solutions to the body.
• Do not grow crystals where food and drink is handled or in bedrooms.
• Take care when handling with hot water and hot solutions.
• Ensure that during growth of the crystal the container with the liquid is out of reach
of children under 10 years of age.
• Goggle markings
– Edu-Science (HK) Ltd 1 S – EN166 3 S H CE
European standard for Personal Eye Protection
Optical glass
S – increases robustness
Manufacturer
3 – for use with liquids
S – intended for protection against droplets or splashes
H – intended for small heads
CE – complies with EN 166
2 3
• Tweezers
• Plastic funnel
• Eyedropper
• Thread
• 15 granite base rocks
• Magnifying glass
• Display stand
• 15 blank labels
• Goggles
(Goggles for supervising
adults are not included)
You will also need
plaster of paris
(not included)
Contents
Table of Contents Important Telephone Numbers
Advice For Supervising Adults

4 5
Introduction To Crystal Growing
In this kit, we show you how to make crystals of different shapes, sizes, and colours.
By experimenting and developing the basic methods, you can create a range of
beautiful crystals. However, before we begin, we need to explain what crystals are.
Millions of years ago, the Earth was not as we know it now, but a mass of constantly
moving hot gases. At some point in time, the gases cooled and formed liquids, some
of which cooled further to become solid materials.
Crystals are solid materials with atoms or molecules that are arranged in orderly,
repeating patterns extending in all three dimensions. Non-crystalline (or amorphous)
materials don’t have this orderly structure. A fundamental property of crystals is their
geometric symmetry. There are 6 different symmetrical shapes used to classify
crystals:
• Cubic – all sides are equal and at right angles to one another
• Tetragonal – this is a cube that has been stretched in one dimension,
so the base is square, but the sides are rectangular
• Monoclinic – the base is a parallelogram instead of square, but the sides
are rectangular
• Triclinic – both the sides and base are formed from parallelograms
• Hexagonal – the base is a hexagon and the sides are rectangular
• Orthombic – similar to a cubic lattice, but one that has been stretched
in two dimensions, so the length, width and height are all different
Our kit uses two types of chemicals for growing chemicals: monoammonium
phosphate, which forms tetragonal crystals, and aluminium potassium sulphate,
which forms monoclinic crystals.
ACTIVITY 1 – Ice Crystals
ACTIVITY 2 – Other Common Crystals
You will need:
• Petri dish
• Water
Method:
Pour a small amount of water into the petri dish and place this in the freezer compartment
of a fridge/freezer. Check regularly for the formation of ice crystals in the dish. Look
closely with your magnifying glass – they should look just like snowflakes!
We’ve included a table on page 12 that you can use to record the results
of your experiments. Doing this is a good way of remembering the
experiments that worked well, or those that didn’t, and the reasons why. You will need:
• Table salt
• Epsom salts
• Clean jam jar
• Pencil
• Paper clip
• Cotton thread
Recipes for salt solutions:
Table salt: 5 tablespoons of salt to 6 tablespoons of water
Epsom salts: 5 tablespoons of Espsom salts to 6 tablespoons of water
Method:
Use a jam jar to prepare one of the two solutions. Add the correct amount of hot water
from the tap to the jar, and then gradually add the table salt or Epsom salts. Add the salt
etc. gradually, stirring well each time, and waiting until it has disappeared before adding
more. Keep adding it until it is not possible to dissolve any more salt in the water, and
there is a small amount left in the bottom of the jar. You have made a saturated solution,
from which you can grow crystals.
Take the pencil and tie a piece of cotton to it, and then attach a paper clip to the free end
of the cotton to hold it down in the solution. Balance the pencil on the rim of the jam jar
with the paper clip immersed in the solution
and cover. Allow to cool and leave
undisturbed for a day or two. Watch
the formation of crystals on the cotton
and check the shape of the crystals
with your magnifying glass.
Tetragonal
Family
Isometric
Family
Monoclinic
Family
Triclinic
Family
Hexagonal
Family
Orthombic
Family
Please note: drawings underneath show the shape of the base of the crystals. Salt crystals growing on string

6 7
ACTIVITY 3 – Preparing a Monoammonium
Phosphate Solution
You will need:
• Orange citrine, Pink quartz, Emerald green, Marine blue, Lilac geode or
Rama quartz crystal growing powders
• Small/large measuring cup
• Clean jam jar
Recipes for crystal growing solutions:
The proportions are:
7cc (12g) powder to 20cc (20g) water
22cc (40g) powder to 70cc (70g) water
Method:
Measure the correct amount of water into the jam jar, and add the corresponding
amount of crystal growing powder. Heat the solution using either a microwave oven
or by placing the jar in a pan of hot water (the level of the water should be the same
as in the jar). DO NOT LET THE SOLUTION BOIL – gradually heat and stir the
solution until the crystal growing powder is fully dissolved. Allow the solution
to cool.
ACTIVITY 5 – Growing a Crystal From a Seed Crystal
You will need:
• Prepared crystal growing solution (from activity 3 or 4)
• Thread
• Pencil
• Plastic container
• Tweezers
• Clean jam jar
Method:
Pour some of the prepared crystal growing solution into one of the petri dishes from the
kit, cover and leave for at least 24 hours. Some crystals should have formed. Using your
tweezers, very carefully pick up one of the larger crystals, dry it on a paper towel and tie
it to a thread. You can do this by making a loop at the end of a piece of cotton and gently
catching the crystal in the loop. You will use this “seed” crystal as a starting point for
growing a larger crystal.
Prepare another solution of the same type, but of a different colour. Attach the other end
of the thread to a pencil and immerse the seed crystal in the solution, with the pencil
balanced on the rim of the jar. Adjust the length of the thread so the crystal is under the
solution but does not touch the walls of the jar. Cover the jar and leave for 12 to 24 hours
– you should see that the crystal is growing.
ACTIVITY 4 – Preparing an Aluminium Potassium
Sulphate Solution
You will need:
•Frosty diamond, Red ruby geode, Pink frost, Yellow geode or Moss geode crystal
growing powders
• Small/large measuring cup
• Clean jam jar
Recipes for crystal growing solutions:
The proportions are:
6cc (10g) powder to 20cc (20g) water
30cc (52g) powder to 100cc (100g) water
Method:
Measure the correct amount of water into the jam jar, and add the corresponding amount
of crystal powder. Heat the solution using either a microwave oven or by placing the jar
in a pan of hot water (the level of the water should be the same as in the jar). DO NOT
LET THE SOLUTION BOIL – gradually heat and stir the solution until the crystal growing
powder is fully dissolved. Allow the solution to cool.
ACTIVITY 6 – Growing Crystals On a Pebble
You will need:
• Prepared crystal growing solution (from activity 3 or 4)
• Granite base stone
• Thread
• Pencil
Method:
Prepare a solution in a jam jar, as detailed in activity 3 or 4. Suspend the granite base
stone in the solution using the thread and pencil. Instead of growing around another seed
crystal, the crystals will grow on the granite base stone.
You may find that crystals form in the bottom of the jar as well as on the pebble. If this
happens, remove the pebble and re-heat the solution until the crystals dissolve. Allow to
cool, then put the pebble back into the jar.
Emerald Green
Crystal
Examples: Examples:
Orange Citrine
Crystal
Lilac Geode
Crystal
Frosty Diamond
Crystal
Red Ruby Geode
Crystal
Moss Geode
Crystal

8 9
ACTIVITY 8 – Making Jewellery/Ornaments
You will need:
• Lids from measuring cups
• Plaster of Paris – not included
• Crystals grown from previous experiments
• Cooking oil (for coating the inside of the lid)
• Eyedropper
• Tweezers
Method:
Coat the inside of the lid with a thin layer of cooking oil to prevent the plaster from
sticking. Place a tablespoon of plaster powder in a small plastic container and use the
eyedropper to gradually add drops of water to the plaster. Use a matchstick to mix the
plaster and water until it has the consistency of a thick pudding. Pour the mixture into the
lid and spread evenly to make the plaster base. Use your tweezers to place the crystal on
to the plaster. Leave for two to three hours until the plaster sets. Gently press the bottom
of the lid to release the plaster cast.
ACTIVITY 7 – Moulded Crystals
You will need:
• Prepared crystal growing solution (from activity 4 or 5)
• Small plastic moulds
Method:
Prepare a solution in a jam jar as detailed in activity 4 or 5. Fill the plastic moulds
with the solution and leave for 24 to 36 hours. The crystals will set in the shape of
the mould. You will need to top up the mould with solution as the crystals grow and
the liquid evaporates. When all of the liquid has evaporated and the crystals are fully
formed, gently take the crystals out of the mould and place on your display stand.
ACTIVITY 10 – Creating Stalactites and Stalagmites
You will need:
• 2 clean jam jars
• Old baking tray or aluminium takeaway dish
• Strip of cotton cloth (preferably terry towelling) 3cm wide x 40cm long
• 12 tablespoons washing soda (sodium carbonate)
Method:
Pour one cup of hot water into each jar and add half of the washing soda to each. Insert
the ends of the cloth into each of the jars, making sure that the ends reach the bottom of
the jars. Place the jars in the baking tray and move the tray to a warm place, well out of
the reach of small children and pets. Wait several days and you will see that the solution
starts to drip and a column begins to form.
ACTIVITY 9 – Making a Crystal Garden
You will need:
• Lids from plastic containers
• Plaster of Paris – not included
• Crystals grown from previous experiments
• Cooking oil (for coating the inside of the lid)
• Eyedropper
• Tweezers
Method:
Prepare a plaster base with one of the lids from the plastic containers. Using your
collection of crystals, embed them into the still soft plaster to create a crystal garden.
Use adhesive to attach crystals, beads and pebbles to the embedded crystals to add
a further dimension to your garden.
Stalactites and Stalagmites
Moulds

10 11
ACTIVITY 11 – Growing Crystals in a Rapidly
Cooled Solution
You will need:
• Prepared crystal growing solution (from activity 4 or 5)
• Granite base stone or seed crystal
• Thread
• Pencil
Method:
Place the container with your solution in a bowl of ice cubes and see what happens.
Record your observations.
ACTIVITY 13 – Growing a Large Crystal
Method:
Try growing as large a crystal as
possible by repeatedly immersing a
crystal in a new saturated solution.
ACTIVITY 12 – Growing Crystals in a Slow Cooled Solution
You will need:
• Prepared crystal growing solution (from activity 4 or 5)
• Granite base stone or seed crystal
• Thread
• Pencil
Method:
Try to make the solution
cool slowly. Either place
the container with your
solution somewhere warm,
or place it in an insulated
(e.g. polystyrene) container
with a lid. Record your
observations.
ACTIVITY 14 – Modelling The Structure of a Crystal
Hints & Tips Section
You will need:
• Models of water molecules (cut out from last page of manual)
Method:
A crystal structure consists of atoms or molecules connected in an organised and
repetitive series, packed together as closely as possible. We can illustrate this using the
example of the water molecule, which consists of two hydrogen atoms and one oxygen
atom, better known by the chemical formula H20. The water molecule looks like this:
• If you are having trouble dissolving the crystal growing powder (salt), try adding a small
amount of water to the solution.
• If a large number of small crystals have formed in the base of the jar, remove the seed
pebble. Reheat the solution and stir until all of the crystals have dissolved. Allow to
cool, and put the seed pebble back in the solution.
• If the crystal is not growing, move the solution to a cooler room.
Cut out the models of the water molecules and try to fit them together as closely as
possible. In figure C opposite, the two ends will not hold together, since both have the
same (negative) charge – they would repel. Likewise, in figure D everything is fine at the
top (since there are positive and negative charges together), but not at the bottom (there
are two positive charges close together). Assemble all of the molecules and check that
everything is correct.
Now get someone else to try. In order to get the molecules as closely together as possible,
you will find that they will make a very similar pattern to your own. This is the basis of
crystal formation – for any par ticular atom or molecule, a par ticular set of rules must be
followed in order to get them to fit together.
The oxygen atom has two negative charges, and each
hydrogen atom a positive charge – they join together to
form a neutral molecule of water. At room temperature,
the water molecules move around quite freely, so they
are not arranged in any particular way. However, as water
is cooled, the molecules move less and less, until they
reach the freezing point, when the molecules are packed
as closely together as possible. Unfortunately, because
the atoms in the molecules have positive and negative
charges, they will only fit together in a certain way –
remember, like charges repel each other.
Rama Quartz Crystal
Marine Blue Crystal
Molecule of water
Yellow Geode Crystal
Example:
Example:
Example:

12 13
Here is an extra page of notes
Chemical Grams ML Date Time Size Time Size Time Size

14 15
Notes: Notes:
Table of contents
Popular Science Education Product manuals by other brands
WATSON INDUSTRIES
WATSON INDUSTRIES VSG-E469 owner's manual

Diffinity Genomics
Diffinity Genomics RapidTip User handbook

3D Molecular Designs
3D Molecular Designs Synapse Construction Kit Assembly instructions

3B SCIENTIFIC
3B SCIENTIFIC U19511 instruction sheet

3B SCIENTIFIC PHYSICS
3B SCIENTIFIC PHYSICS 1017735 instruction manual
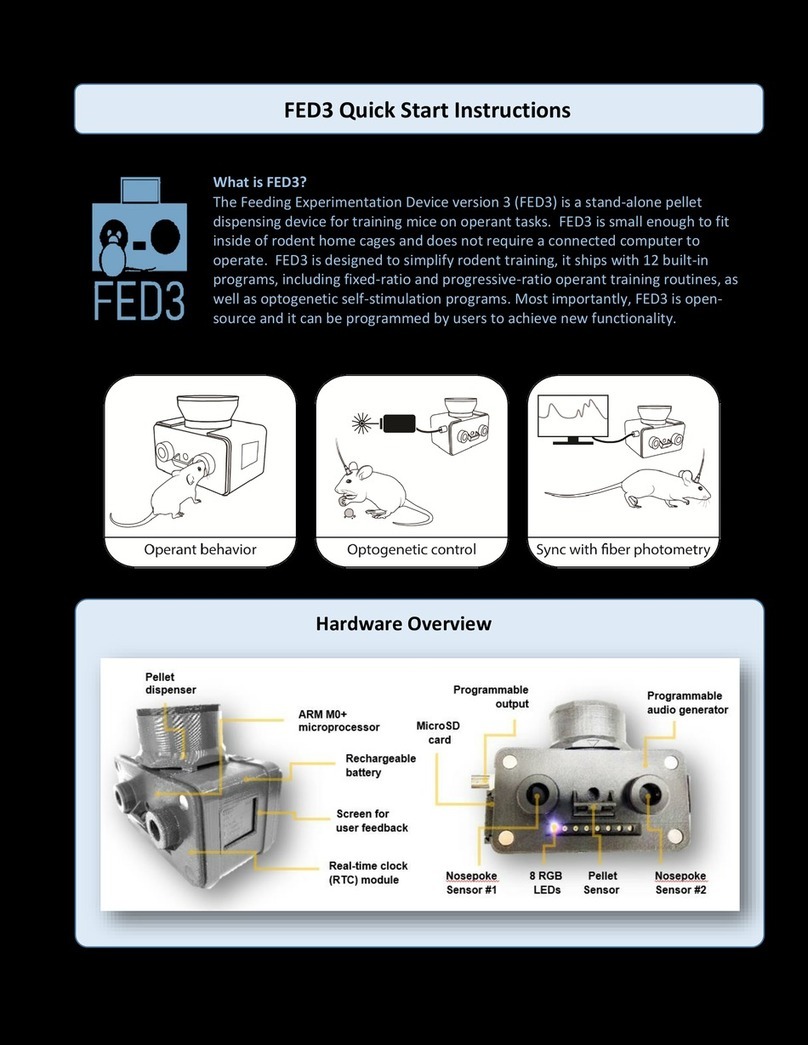
HACKADAY
HACKADAY FED3 Quick start instructions
Life form
Life form LF03699U instruction manual

DAGU
DAGU Arexx SOLAR E CAR JSR-SC2 instruction manual

3D Molecular Designs
3D Molecular Designs ENZYMES IN ACTION KIT 6-Group Set manual

DEWESOFT
DEWESOFT VIBRO KIT V20-2 Technical reference manual

YUKI MODEL
YUKI MODEL Gyro Hero2.0 How-to

Littlebits
Littlebits Space Kit Version 1 manual