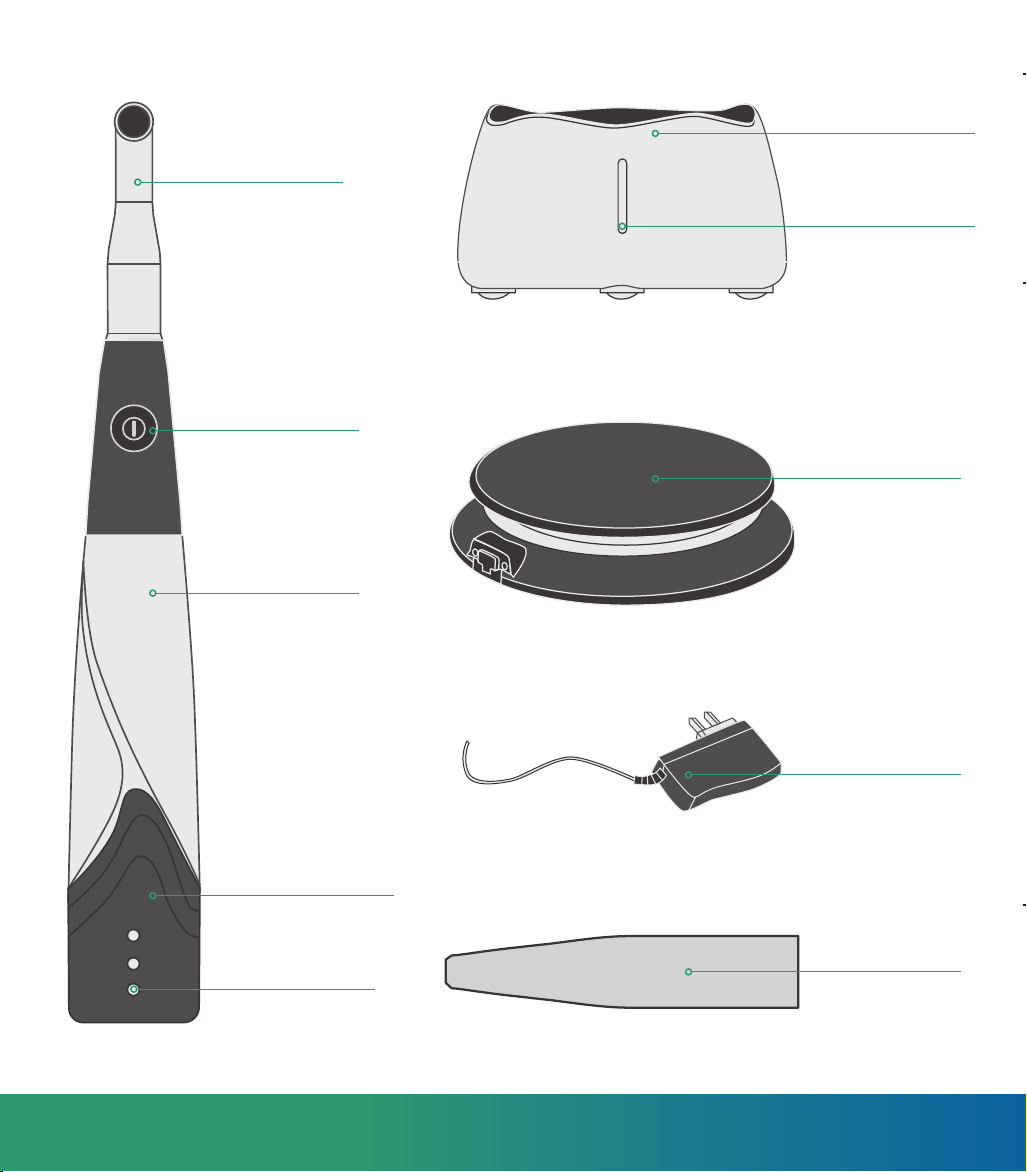
6
• Doctors with a pacemaker are prohibited from using this device.
• Patients with a pacemaker (or other electrical equipment) who are warned not to use small appliances (such as
Electric razors, hair dryers, etc.) are prohibited from using this device.
• Hemophilia patients are prohibited from using this device.
• For patients with heart disease, pregnant women and young children, cautiously use this device.
For patients or users with allergy concerns, refer to product allergen document available at www.ultradent.com. If
allergic reaction is observed, rinse exposed area thoroughly with water and have the patient consult their physician.
• Do not place device near a heat source.
• Operate and store device in a safe dry place.
• Do not use lubrication on the motor component.
• Use caution for patients with heart conditions, pregnant women, and children.
• This device requires special precautions regarding electromagnetic compatibility (EMC) and must be in strict
accordance with the EMC information for installation and use. Do not use this equipment near uorescent lamps,
radio transmitting devices, remote control devices, handheld or mobile high-frequency
communication devices.
• Long term use of equipment may result in overheating of the motor handpiece and should be left to cool until
next use. Please contact Ultradent if any issues arise.
• To avoid damage or malfunction of device, use the original charging base.
• Any changes to the device may violate safety regulations, causing harm to patients. The manufacturer will not
accept any liability for a modied device.
• To avoid damage to lithium battery and control circuit, use original power adapter.
• If you think a battery replacement is necessary, please contact Ultradent. Do not attempt to replace the battery.
• To avoid damage, ensure that the device is in the o position before removing or installing the outer sheath or
disposable prophy angle.
• To avoid damage to the handpiece and disposable prophy angle, only use disposable prophy angles compatible
with this device and be sure that they are correctly installed before starting the handpiece.
• Wireless charging will generate heat. The surface temperature of the charging station and motor handpiece will rise.
• According to IEC 60601-1/UL60601-1, this device must not be used in the presence of a ammable anesthetic gas
mixed with air, oxygen, or nitrous oxide. (NOTE: Nitrous oxide by itself is not a ammable anesthetic gas.)
• Do not use handpiece while charging.