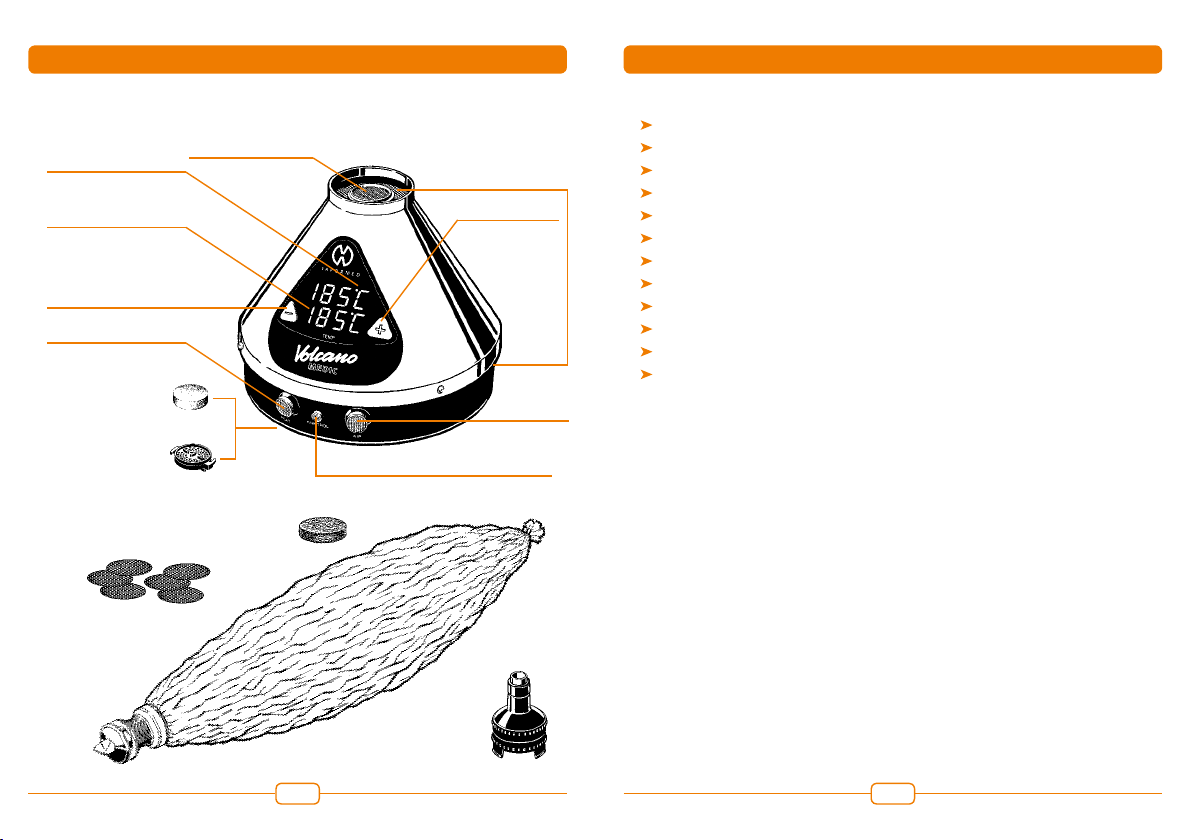
REF
IP 21
0123
1110
EU conformity symbol
A 4-digit number after the CE marking indicates that a notified
body is involved in the conformity assessment procedure.
Safety tested and production monitored by TUEV SUED according
to IEC 60601-1, CAN/CSA C22.2 No. 60601-1.
Device protected against foreign bodies with a diameter larger
than 12 mm and against vertically falling water drops (according to
IEC 60529).
Symbol for order number – followed by the order number of the
corresponding medical product (or part)
2. EXPLANATION OF SYMBOLS, SAFETY
RECOMMENDATIONS
Contains or presence of phthalate
Air pressure limitation
Caution! Hot surfaces!
Never leave Filling Chamber on the VOLCANO MEDIC Hot Air
Generator.
Keep away from sunlight
Protect against moisture and humidity
Interference may occur in the vicinity of equipment marked with
the following symbol.
Temperature limits of ambient temperature
Humidity limitation
Safety Recommendations
The packaging material (plastic bags,
styrofoam material, boxes, etc.) must
be kept out of the reach of children as
it is a potential source of danger.
People who require assistance must
be supervised constantly during
inhalation. Such persons often
underestimate the hazards involved
(e.g. strangulation with the power
cord), so resulting in injury.
The device contains small parts
which can block the respiratory tract
and lead to choking. Therefore, make
sure that you always keep the Hot Air
Generator and accessories out of the
reach of babies and infants.
Before connecting the Hot Air Gen-
erator, make sure that the information
given on the type label on the bottom
side of the Hot Air Generator cor-
responds to the mains supply at the
place of installation.
If there are any problems during
operation, immediately pull out the
power plug from the socket.
The power cord must be unwound
over its entire length (avoid rolling up
and overlapping the cord). It must not
be exposed to any impacts and must
be kept out of the reach of children.
Also, it must not be kept near liquids
or sources of heat and must not be
damaged.
Do not wind the power cord tightly,
nor pull it over sharp edges and never
crush or kink it. If the power cord is
damaged, please contact our Service
Center to have it replaced. Never try
to repair a power cord yourself!
The use of multiple sockets and/or
extension cables is not recommend-
ed. However, if this is absolutely
necessary, only products with a
quality certificate (such as UL, IMQ,
VED, +S etc.) may be used, provided
the specified power exceeds the
power required (A= Ampere) by the
devices that are connected.
If you have any concerns, ask a
specialist to check whether the
electrical system is in accordance
with the local safety regulations.
Install the Hot Air Generator on a
stable and dry surface at a sufficient
distance from sources of heat
(oven,stove, fireplace etc.) and in a
place where the ambient tem-
perature cannot drop below +5°C
(+41°F). Store the Hot Air Genera-
tor in a dry place protected against
the effects of weather and out of
the reach of children or unqualified
persons. It must not be installed in
damp locations (such as bathrooms
etc.).
The Hot Air Generator may only be
repaired by our Service Center. In
correctly performed repairs without
the use of original spare parts can-
prove dangerous to the user.
2. EXPLANATION OF SYMBOLS, SAFETY
RECOMMENDATIONS