ENGLISH • 3
Introduction/Indications for Use ............................................................................................................4–5
Care and Safety Information..................................................................................................................6–13
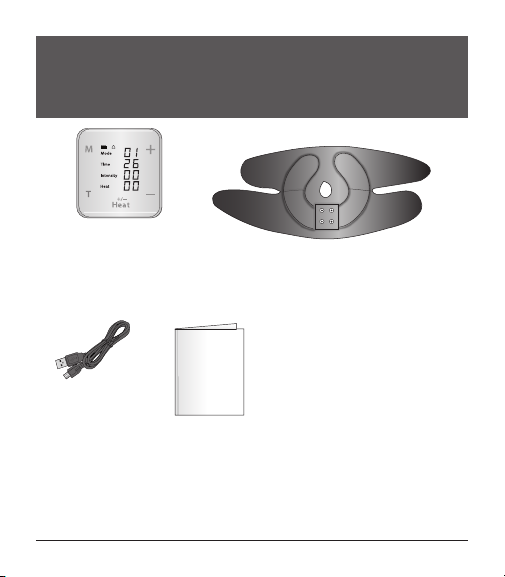
Product Features.................................................................................................................................... 14–15
Charging the Device ............................................................................................................................. 16–17
Preparing Your Device .......................................................................................................................... 18–20
Placement & Treatment............................................................................................................................... 21
Pulse Program Descriptions................................................................................................................ 21–22
Instructions for Use............................................................................................................................... 23–26
Optional Accessory —Gel Pads ......................................................................................................... 27–30
Cleaning and Storage.................................................................................................................................. 31
Troubleshooting ........................................................................................................................................... 32
Device & Label Symbols.............................................................................................................................. 33
Electromagentic Compatibility.......................................................................................................... 34–37
Product Specications.......................................................................................................................... 38–39
Warranty Information........................................................................................................................... 40–41
Español..................................................................................................................................................... 43–83
Toll-Free Customer Care Help Line:
1-866-326-1313
Monday – Friday
8:30 a.m. – 4:30 p.m. CST
INDEX
Distributed by
Veridian Healthcare
1175 Lakeside Drive
Gurnee, IL 60031
www.veridianhealthcare.com
Made in China
#93-1371 01/18
©2018 Veridian Healthcare, LLC