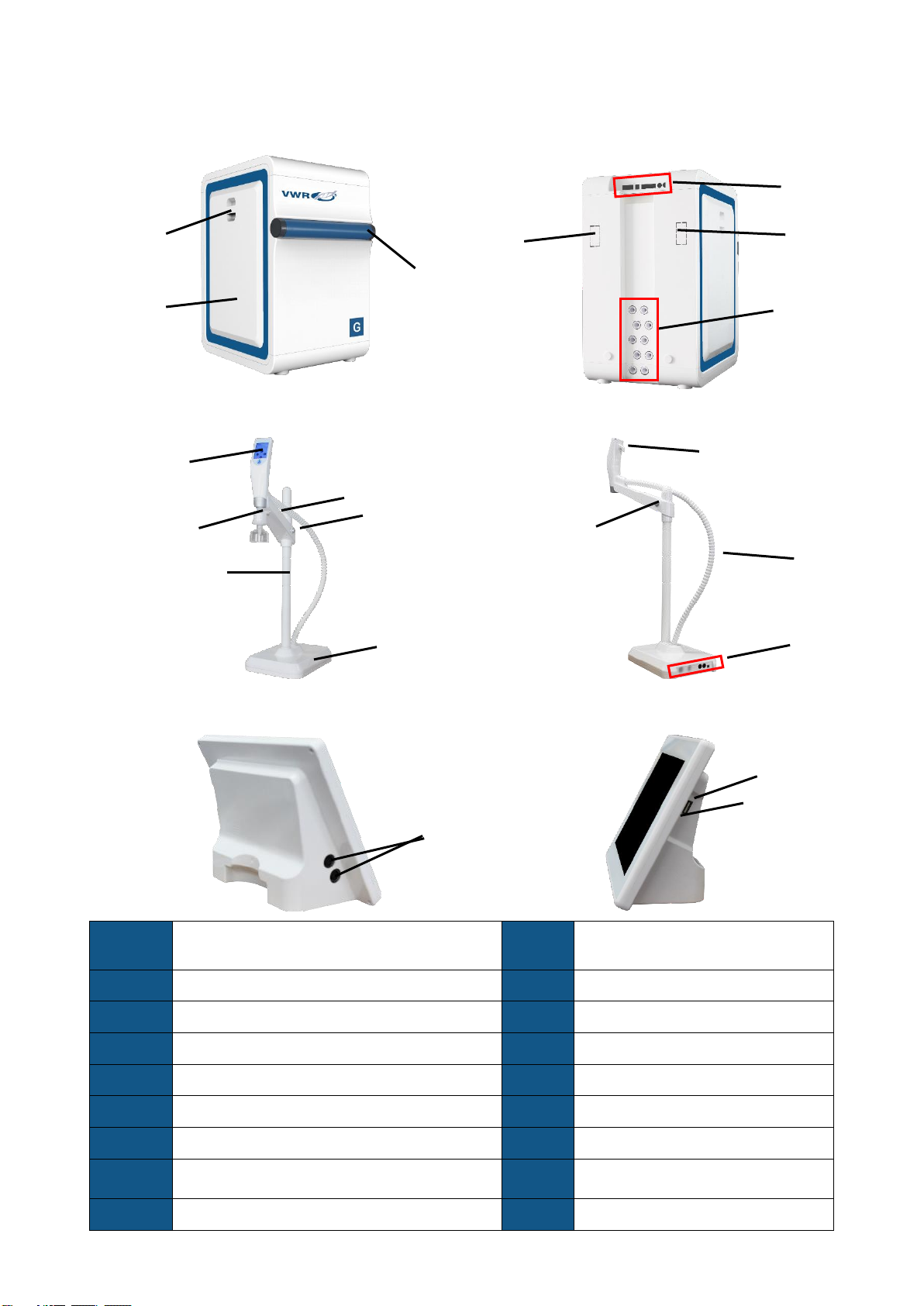
7vwr.com I Instruction manual VWR® G Water System
1.2 Product Features
The VWR G ultrapure water system provides an integrated solution for lab water supply. This
system is easy to install, easy to operate and easy to maintain.
This system has the following features:
◆Type I ultrapure water and EDI (Type II) water are produced directly from tap water.
◆The system can be linked to multiple dispensers via cable or wireless.
◆Internal P Pack cartridge removes oxidants, organics, particles and scaling ions to prevent
them from fouling the RO membranes and the EDI module.
◆Alarm will lit if RO permeate does not meet preset quality standard (SP) to protect the EDI
module.
◆The RO drain water is reused to increase the water yield. It is more environmentally friendly.
◆Internal Ion-Pure EDI module removes most ions and organics from RO water.
◆Ultra purification cartridge is filled with media to remove trace ions and organics.
◆Built-in 254 nm lamp kills bacteria and 185/254 nm dual-wavelength lamp reduces TOC level
in ultrapure water.
◆On-line TOC monitor is based on complete oxidation method to obtain a more accurate
measurement of the TOC.
◆Various final filters (optional) can ensure ultrapure water without particles, bacteria or
pyrogen.
◆Tank recirculation mode (optional) from a touch on the monitor guarantees high water quality
in storage.
◆RFID tags ensure perfect placement of consumables and trace their performance.
◆The control console is an 8-inch touchscreen. The console controls system and peripheral
devices (such as dispensers and tank sanitization module). All operations can be done on
the console.
◆Water quality, operation parameters, the status of the system, dispensers, components, and
peripheral devices are stored and displayed on the large colour touchscreen monitor.
◆The console screen and dispenser screens are water-proof. You may operate the console
and dispenser with wet latex gloves on.
◆Signature verification is required for maintenance and service.
◆VWR provides full documents support to meet user’s GMP, GSP, GAP, GCP, GLP
requirements.