Do not refrigerate or freeze test strips.
Do not store or use strips in a humid environment, such as a bathroom.
Do not store the meter, test strips, or control solution near bleach or
cleaning agents that contain bleach.
Close the cap on the vial immediately after removing a test strip.
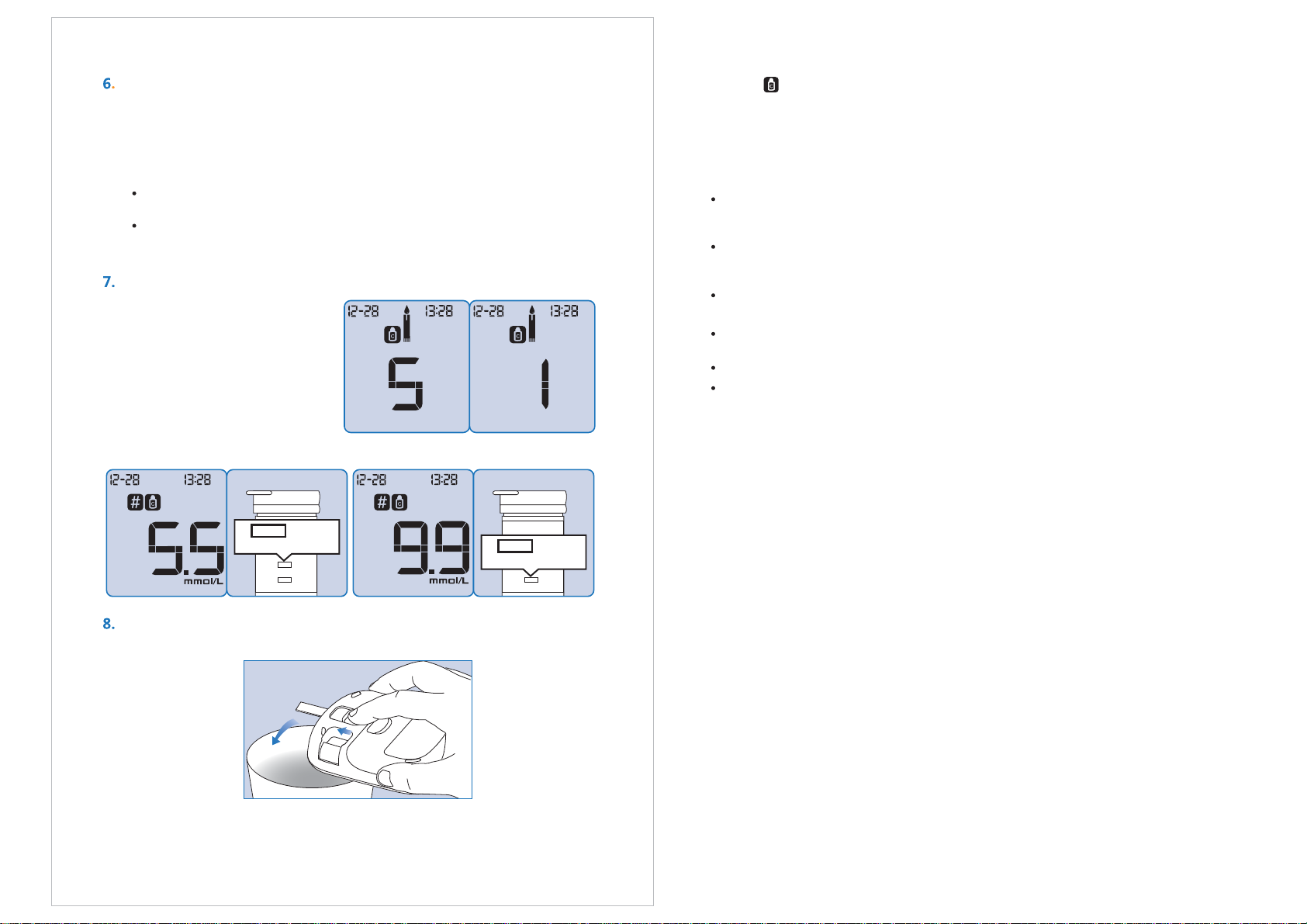
Use the test strip immediately after removing it from the package.
Do not use expired test strips. Doing so may lead to inaccurate results.
Tip: The test strip label contains the expiration date in year-month format. For
example, 2017-01 indicates that the test strips are valid until January 2017.
Special Instructions for Test Strips Sold in a Vial
Test strips should be stored in the tightly capped vial that is provided.
Do not store test strips outside of the provided vial. Test strips must be
stored inside the original vial with the lid tightly sealed closed.
Do not transfer test strips from the provided vial into another container.
Close the cap on the vial immediately after removing a test strip.
A new vial of test strips may be used for 6 months after first being
opened. Please take note of the date that the vial was first opened,
and discard after 6 months.
Special Instructions for Test Strips Sold in
Single Use Packets
Tear the packet open carefully beginning from the tear gap. Do not
damage or bend the test strip.
Use the test strip immediately after removing it from the packet.
Test Strip Precautions
For in vitro diagnostic use.
Use the test strip immediately after removing it from the package,
otherwise the test results may not be accurate.
Do not use test strips that are torn, bent, or damaged in any way. Do
not reuse test strips.
Keep the test strip packaging away from children and pets.
Consult your physician or healthcare professional before making any
changes in your treatment plan based on your blood glucose test
results.
Please refer to the test strip instructions for more detailed information.
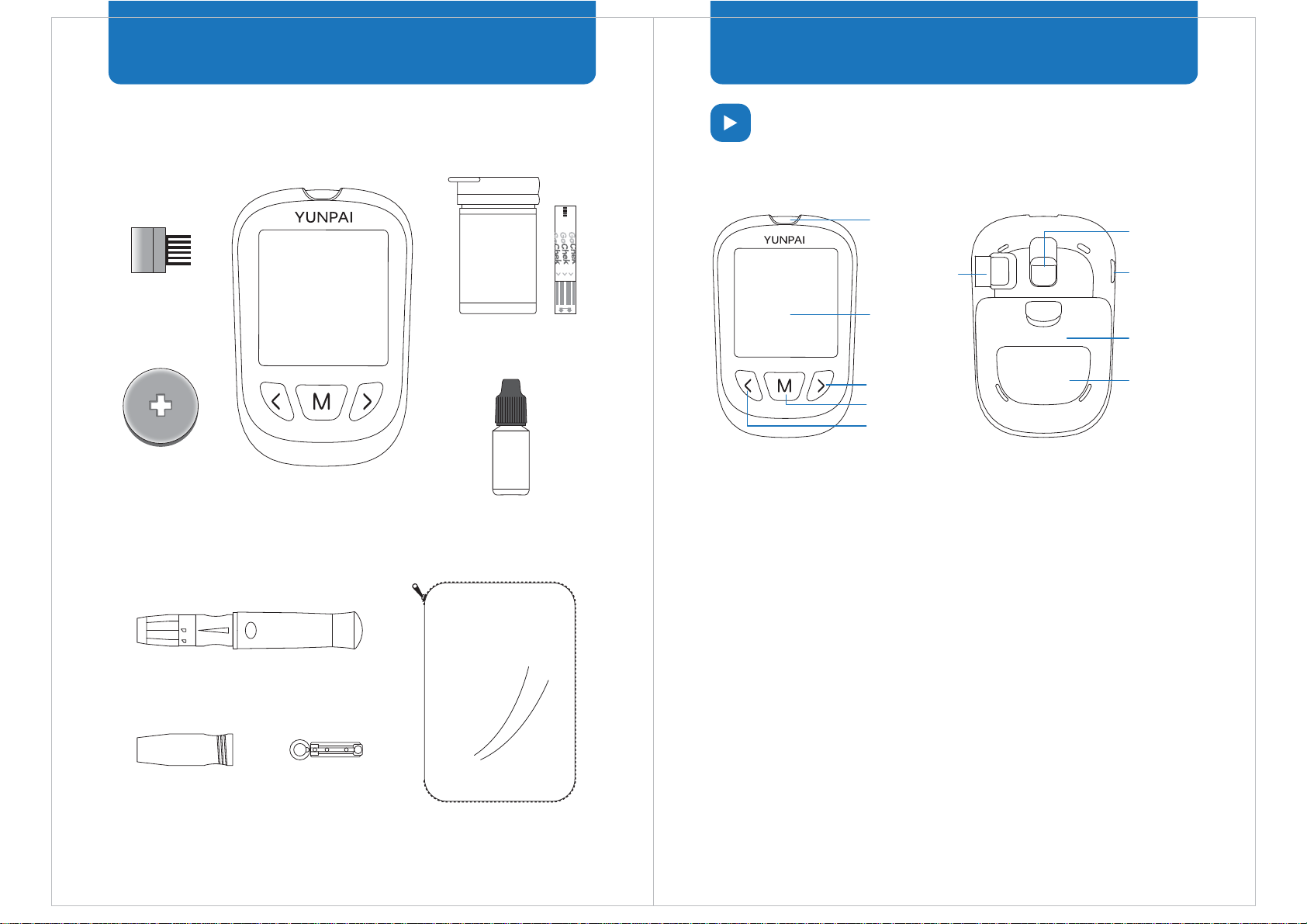
Code Chip
Insert the code chip into the meter before each test. It is used to
calibrate the meter for use with the associated test strips.
Control Solution
Control solution is a glucose solution of known concentration that is used
to confirm that your meter and test strips are working properly. It is
important to run a quality control test regularly to make sure that you are
getting accurate results.
You should perform a quality control test in the following situations:
Refer to page 12 for instructions on how to perform a quality control test.
Storage and Handling
Please review the following storage and handling instructions:
Tip: The control solution label contains the expiration date in year-month format. For
example, 2017-01 indicates that the test strips are valid until January 2017.
When you suspect that the meter or test strips
are not working properly.
When you suspect that your test results are
inaccurate, or inconsistent with how you feel.
When you suspect that your meter has been
damaged.
After cleaning your meter.
Store the control solution in the temperature range 5-30°C (41-86°F).
Do not refrigerate or freeze the control solution.
If the control solution is cold, do not use until it has warmed to room
temperature.
Do not use expired control solution.
Control solution may be used for 6 months after the bottle is opened
for the first time. Please take note of the date that the bottle was first
opened, and discard after 6 months. Do not use beyond the expiration
date.
Control Solution Precautions
For in vitro diagnostic use. The control solution is for testing only
outside of the body. Do not swallow or inject.
Control solution should be shaken before use.
Quality control tests should be carried out at 15-30°C.
Do not let the control solution bottle touch the test strip.
Use only the control solution that is recommended for your meter.
The control ranges shown on the test strip package are not
recommended ranges for your blood glucose level. Your personal
glucose range should be determined by your healthcare professional.