CONTENTS
GENERAL DESCRIPTION................................................................... 1
SYSTEM COMPONENTS ................................................................... 1
WARRANTY .............................................................................. 2
INDICATIONS AND CONTRAINDICATIONS ..................................................... 2
WARNINGS AND PRECAUTIONS ............................................................ 4
ABOUT THE DEVICE....................................................................... 6
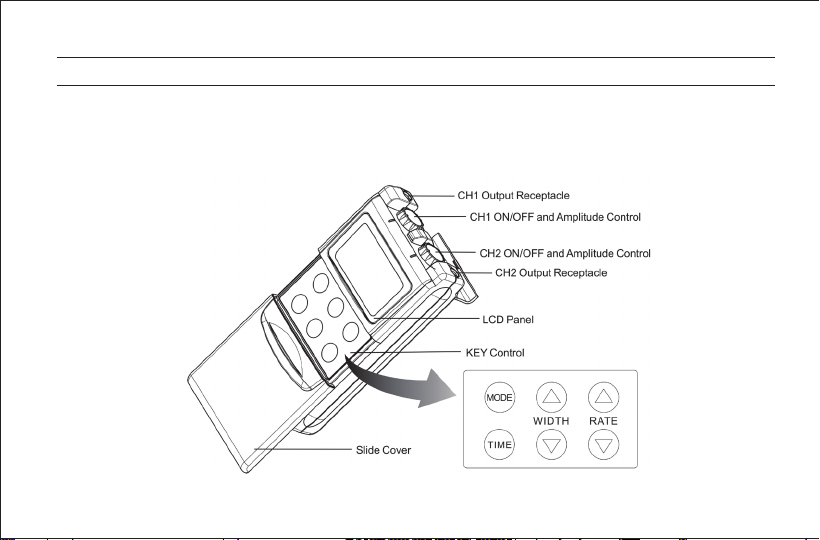
THE DEVICE CONTROLS................................................................... 7
DANGER ................................................................................ 8
ATTACHING THE LEAD WIRES .............................................................. 9
ELECTRODE SELECTION AND CARE......................................................... 9
TIPS FOR SKIN CARE......................................................................10
CONNECTING THE DEVICE.................................................................11
BATTERY INFORMATION ...................................................................12
CHANGING THE BATTERY..................................................................13
CLEANING FOR YOUR DEVICE..............................................................13
TROUBLESHOOTING ......................................................................14
TECHNICAL SPECIFICATIONS...............................................................15