Apex Digital Mobi Mesh User manual

Mobi Mesh
Instruction Manual


CONTENTS
USER’S MANUAL 1
MANUAL DE INSTRUCCIONES 15
INSTRUÇÕES DE UTILIZAÇÃO 30
INSTRUCTIONS D'UTILISATION 45
GEBRAUCHSANLEITUNG 61
GEBRUIKERSHANDLEIDING 77
MANUALE DI ISTRUZIONI 93
MODEL NO:9R-001
PLEASE READ ALL INSTRUCTIONS BEFORE USE.

1
Mobi Mesh / English
Dear Customer,
This manual contains important information about the product. Please read it thoroughly before use, and
keep this manual. The Nebulizer’s flow rate will vary depending on what medicine is used; in addition, when
the medicine is at a low temperature the flow rate will decrease.
INTENDED USE
Medical purpose - Intended user
This product is intended to be used for inhaling medication for respiratory disorders by legally certified medical
experts, such as doctors, nurses and therapists, or healthcare personnel or patients under the guidance of qual-
ified medical experts.
Environment
This product is intended for use in a medical facility, such as a hospital, clinic or doctor’s office, in a room in a
house or in an open-air environment with a roof.
Warranty
The Mobi Mesh has a two-year limited warranty (with exception of the mouthpiece, medical cup and mask) as
free from defective workmanship from the date of purchase. Any defective part(s) will be repaired or replaced by
APEX Medical if the unit has not been tampered with or used improperly during that period. Make certain that any
malfunction is not due to inadequate cleaning or failure to follow the instructions. If repair is necessary, contact
your authorized service personnel for instructions.
NOTE - Be sure to retain a dated proof of purchase document to prove that the unit is within the 2-year warranty
period.
NOTE -This warranty does not cover providing a loan unit or compensation for costs incurred in rental while the
unit is under repair.
The single patient use accessories are expected to last for two years (shelf life) and six months (service life).

2Mobi Mesh / English
Precautions
Warnings described in the instruction manual should be observed.
• The mask system does not contain DEHP.
IMPORTANT SAFETY INSTRUCTIONS
READ THIS BEFORE USING
When using an electronic appliance, basic precautions should always be followed, including the following.
WARNING:
1. Close supervision is necessary when this appliance is used by, on or near children, handicapped people or
invalids. In addition, the dosage and frequency of use also has to be specified by the prescribing physician.
2. Do not operate under a blanket or pillow. Overheating can occur and cause fire, electric shock or injury.
3. Use this appliance only for its intended use as described in this manual.
4. Do not use cotton swabs or other objects to touch the mesh; otherwise, the product will not operate.
5. Do not use different types of batteries at the same time.
6. Do not carry or store the product when there is still medicine in the medical cup.
7. For the type, dose and regime of the medication, follow the instructions of a doctor.
8. Do not immerse in water.
9. Do not use any disinfection solution containing sodium hypochlorite.
10. Do not inhale using water in the medical cup.
11. Do not drop or apply force to the main unit.
12. D o not use any attachments or accessories not recommended by the manufacturer.
13. Discard the waste batteries and device according to local law.

3
Mobi Mesh / English
14. Do not use the device on top of or close to other electronic devices. FEATURES:
15. This product utilizes piezoelectric vibration to turn a liquid drug into a fine mist, which is then sent to the
respiratory tract and oral cavity by a venting system so that therapeutic results can be achieved.
16. Piezoelectric vibration produces 100,000~120,000 vibrations per second.
17. Compact and easy to carry.
18. After 20 minutes continuous operation or when the medical cup has no medicine in it, the appliance will
automatically shut down. However, the no-medication detector may not function if the mesh is blocked by
medication.
19. Built-in low-battery indicator.
20. Even if the product is used at a different angle, it still can operate normally for a short time. When the product is
turned upside down and the medicine cannot make contact with the mesh, the product still can operate normally
for a few seconds (this can differ according to the type of medicine).
NOTICE FOR SAFE OPERATION
To ensure safety, please follow all the rules below:
1. Before using
• Use this appliance in the correct manner outlined in this manual.
• This appliance must be operated within the temperature of 10ºC ~ 40ºC; the product should not be operated
outside this temperature range.
• The medical treatment should be prescribed by a doctor.
• If the product does not work properly, please discontinue use and send it for repair immediately or consult your
dealer.
• If the medical cup is not functioning well (such as very low flow rate....etc.), before using, please replace it.
• When this appliance has not been used for a while, if any part (such as the medical cup, mouthpiece or mask)
seems dirty, please proceed with cleaning procedure described in the cleaning and maintenance procedure.

4Mobi Mesh / English
• Do not add medicine over the indicated line marked on medical cup (8cc marking).
• Do not disassemble or make any modification to this appliance.
2. When using
• Children or disabled patients should be assisted by adults or medical personnel when using this appliance.
• Do not use this appliance in a highly humid environment (>85% RH)
• Do not use this product if there is no medicine in the medical cup.
• Keep the appliance in a horizontal position and steady during operation to avoid spillage.
• Do not open the cover of the medical cup during operation.
• Keep eyes away from the mist output.
• Treatment period: please follow the doctor’s advice.
• Different patients should use individual clean medical cups and mouth pieces to avoid cross-infection.
• If some medicines do not nebulize well, please consult your doctor.
• This appliance has a low-battery indicator. When the indicator light is green, the power is good. When the power light
turns to orange, the battery has become low, but you can still use the product, although you may need to replace the
battery soon. If the power light turns red it means there is no power, and you should replace the battery immediately.
• While using this appliance, if any irregularity or discomfort is observed, please discontinue use of this appliance.
• This product has a built-in timer which shuts off automatically after 20 minutes. If it cannot be shut off automatically,
discontinue use and consult your dealer for help.
• If the device detects no medicine in the medical cup, the LED indicator will turn red and flash three times and then
shut off automatically.
• The device can be used at any angle. However, make sure the medicine has good contact with the mesh. Please clean
all parts after each usage and follow the instructions in the manual. Do not immerse this appliance in water when
cleaning to avoid damaging any electrical parts.
5
Mobi Mesh / English
WARNING:The turbo mode is for self-cleaning purposes only; it cannot be used in normal daily
treatment. Please consult your local distributor for further instructions.
3. After using
• Please clean all parts after each usage and follow the instructions in the manual.
• Do not immerse this appliance in water when cleaning to avoid damaging any electrical parts.
• Do not store this appliance in direct sunlight, outside our suggestion storage temperature or in a humid environment
• Keep this product out of the reach of children.
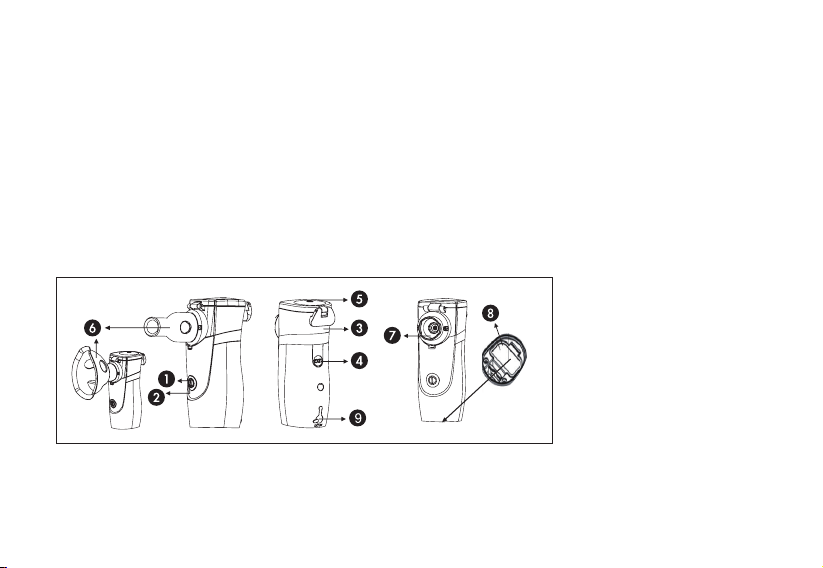
COMPONENTES
1. Power button: Press to turn the appliance on or off.
2. Indicator Light: Indicates the power condition. (Green for normal/Orange for
low/Red for no power). If there is no medicine inside, the indicator light will turn
red and flash three times before turning off automatically. However, the no-
medication detector may not function if the mesh is blocked by medication.
3. Medical Cup: Contains the
medicine. (Suggestion:
Replace with a new medical
cup every year)
4. Push button: Push here to
release the medical cup
from the main body.
5. Medical cup cover: Open
here to pour in the medicine.
6. Mouthpiece or Mask.
7. Mesh
8. Battery cover: Open here to
replace batteries.

6Mobi Mesh / English
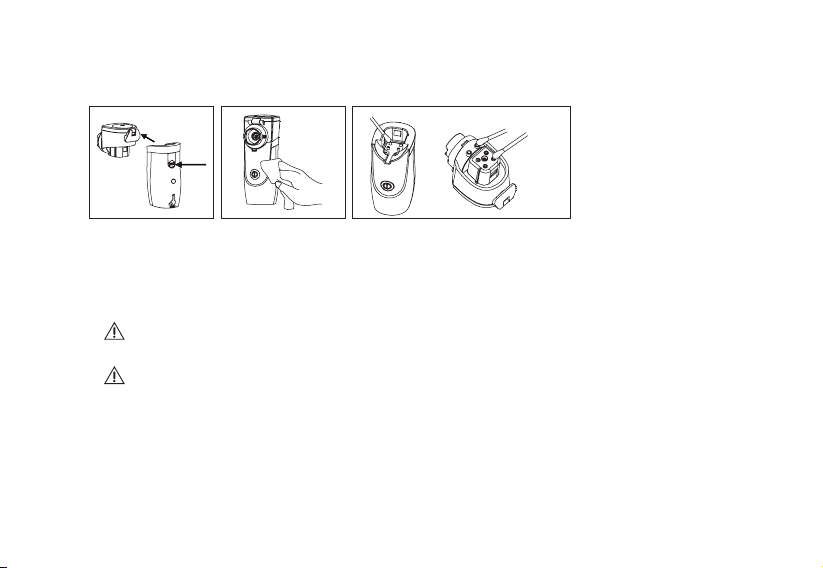
HOW TO USE
1. Make sure that all parts, such as the mouthpiece,
mask and medical cup are clean, or clean these
three parts before using.
2. Open the battery cover and put in the batteries.
(The battery life varies with different brands of
battery)
1. Remove batteries if the
unit is not in use for long
periods. Failure to do so
could result in damage
due to battery leakage.
2. Discard the waste
batteries according to
local law.
3. Open the medical cup
cover, pour the medicine
into the medical cup
and close the cover
completely.
4. Install the mask or
mouthpiece properly and
start using the appliance.
32
1. When the power button is pressed and the appliance
starts working, the green light will turn on (sometimes
this will be an orange light, depending on battery power
remaining) and the built-in timer will start to count down.
2. If the indicator turns orange, it shows the battery has
low power. If the appliance is activated and the power
indicator turns red, it means the batteries have no power
and the appliance cannot operate properly. Please
replace with new batteries immediately.
3. If there is no medicine inside the medical cup, the
indicator light will turn red and flash three times before
the appliance turns off automatically.
Main BODY
Mask Mouthpiece
Medical cup
7
Mobi Mesh / English
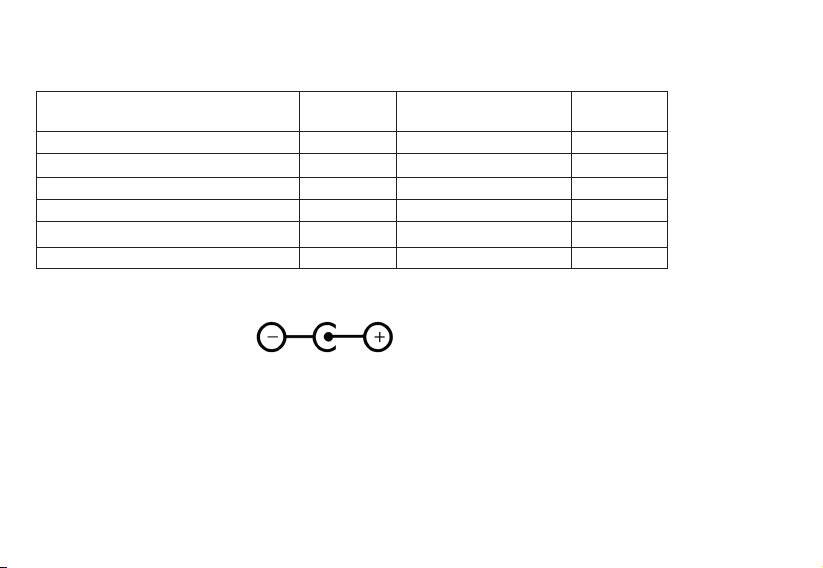
CLEAN AND MAINTENANCE NOTICE
Before and after each using, please clean the following parts:
123
1. Remove the medical cup and mouthpiece or mask.
2. Discard all remaining medicine in the cup.
• Open the medical cap and discard all remaining medicine.
• Pour in some hot water and close the cover completely.
• Press the power button and operate 1~2minutes to discard all remaining medicine completely.
Ensure medicine be cleaned completely after each using, otherwise, mesh can be blocked.
3. Clean the surface of main body of appliance with cotton swabs or a piece of fine cotton fabrics.
Please clean the electrode in the main body and medical cup, ensure normally electrical conduction.
Please do not use easily volatility liquid to clean the electrode, ex.benzene, thinner.
4. Clean the medical cup, mouthpiece and mask with clean water and store at a safety place after cleaning the
surface with ethanol.
Suggestion: Use a new medical cup for different illness or patients to avoid cross infection.
DISINFECTION
Medical cup can be disinfected by boiling in hot water (90°C):1 min, Validated number of cycles are 20. After 1 minute
boiling, shake off excessive water from the cup, and let dry on a clean, lint free(paper) towel. Main unit, mask, mouth
piece cannot be boiled.

8Mobi Mesh / English
TROUBLE SHOOTING
After checking the followings, if any problem still persists, please return the appliance to your
dealer for servicing.
Q1. Press the power button, but no operation
1. Check if the battery voltage is sufficient? When the appliance’s red indicator light is on,
the battery is no power. Please replace the battery immediately.
2. Discard the waste batteries according to local law.
• Check the battery is installed correctly?
Open battery cover and check if the battery electrode is rusted or not.
Q2. Cannot nebulize or not enough mist output
1. Check if the medical cup is filled with appropriate amount of medicine?
2. The mesh in medical cup blocked by remaining medicine. Cleaning medical cup, if still can’t solve problem,
please replace a new medical cup.
3. The mesh surface(mesh output)has covered by water or medication. Remove water or medication from mesh
surface.
4. Medical cup is not installing properly. Release the medical cup from main body and push it back again.
Suggestion : Replace a new medical cup per year.
9
Mobi Mesh / English
THE ACCESSORIES
Item Mode no. Size Single patient
reusable
Child Mask 9R-001MS 87.22 x 64.35 x 57 mm YES
Adult Mask 9R-001ML 124 x 77 x 68.5 mm YES
Mouth Piece 9R-001MP 43 x 27.5 mm YES
Medical Cup (Gray) 9R-001MCG 54 x 43.99 x 38.50 mm YES
Adaptor (USA),(not included) 9R-AU 90 x 76 x53 mm N/A
Adaptor (EU),(not included) 9R-AE 90 x 76 x53 mm N/A
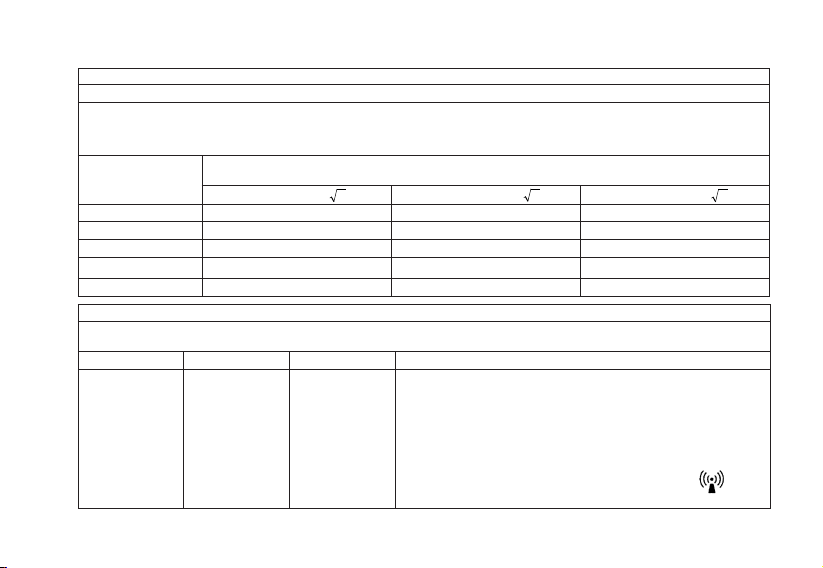
Note: In order to provide optimal performance and prevent device damage, please use our Genuine AC adaptor.
* Our adaptor polarity is represented by the following symbol.
ELECTROMAGNETIC COMPATIBILITY
If other electronic devices are in use around you when the medical device is in use, it may be susceptible to electromagnet-
ic interference from these devices. Electromagnetic interference may cause incorrect operation of the medical device and
could generate an unsafe situation. In order to prevent unsafe situations, the device is designed and tested to standard EN
60601-1-2, which defines the levels of immunity to electromagnetic interference as well as maximum levels of electromag-
netic emissions for medical devices. Even though the electromagnetic emissions of the device are extremely low, special
precaution still need to be observed:
• It is recommended that you do not place the device on top of or close to other devices (such as mobile phone). Should
you notice any interference with other electrical devices, keep the device away from those electronic devices.

10 Mobi Mesh / English
SYMBOLS DESCRIPTION告告告
Class II
Attention, should read the instructions.
Protected against solid foreign objects of
12,5 mm and greater; Protection against
vertically falling water drops .when the
enclosure is tilted at an angle up to 15°
from its normal position.
BF symbol, which indicates that this prod-
uct complies with degree of protection
against electric shock for type BF equip-
ment.
Direct current
Read the documentation symbol
Batch Symbol (yyymmddxxx) yyy:ROC cal-
ender year,mm:month,d:day,xxx:lot no.
Serial number Symbol
Manufacturer
Authorised representative in the European com-
munity.
Refer to instruction manual
CE mark and notified body registration number,
the Annex II, excluding Section 4 of EC directive
93/42/EEC have been met.
Reference number
Disposal of Electrical & Electronic Equipment
(WEEE):
This product should be handed over to a suitable
collection point for the recycling of electrical and
electronic equipment. For more detailed informa-
tion about the recycling of this product, please
contact your local city council, household waste
disposal service or the retail store where you pur-
chased this product.
Temperature limitation/temperature range
Humidity limitation/Humidity range

11
Mobi Mesh / English
Model Number 9R-001
Power Supply 1.5VX 2, AA size alkaline battery
AC adaptor (Input: 100~240VAC, 0.3-0.15A, 50-
60Hz/ Output: 3VDC, 1.33A)
Power Consumption < 2W
Vibrating Frequency 103~123 kHz ±1K
Battery life Up to 1.5 hr if use continously
2 weeks if daily use for 6 min treatment cycle
Battery Indication Full (green), Low (orange), No power (red)
Flow Rate 0.35 ±0.1 mL/min
Particle Size <5μm
AUTO-OFF Time 20 min
Nebulizer AUTO-OFF No medication and no power
Medical Cup Volume 8mL (max.)
Weight 138g (excluding battery)
Dimension 44.5mm (L) x 53.4mm (W) x 121.8mm (H)
Medical Cup Volume 8mL (max.)
Accessory Medical cup, mask (adult & child), mouthpiece
Operation environment 10~40°C, 30~80%RH
Storage/Transportation -20~70°C, ≤85%RH
Sound Noise level (at 1m distance) 50 dB
Performance may vary with drugs such as suspensions or with
high viscosity.
More information can be obtained from the relevant medicine
manufacturer.
TECHNICAL DATA
12 Mobi Mesh / English
Vapor Expert (PY-001) Portable Nebulizer - Instruction Manual
Recommended separation distances between portable and mobile RF communications equipment and the ME equipment
The ME equipment is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The
customer or the user of the ME equipment can help prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and the ME equipment as recommended below, according to the
maximum output power of the communications equipment.
Rated maximum output
power of transmitter
W
Separation distance according to frequency of transmitter m
150 kHz to 80 MHz d=1,2
P
80 MHz to 800 MHz d=1,2
P
800 MHz to 2.5 GHz d=2,3
P
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
The ME declaration – electromagnetic immunity
The ME system is intended for use in the electromagnetic environment specified below.
The customer or the user of the ME system should ensure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
3V
3V/m
Portable and mobile RF communicationss equipment should be used no
closer to any part of the EQUIPMENT or SYSTEM including cables, than
the recommended separation distance calculated from the equation
applicable to the frequency of the transmitter. Interference may occur in
the vicinity of equipment marked with the following symbol.

13
Mobi Mesh / English
Declaration – electromagnetic immunity
The ME system is intended for use in the electromagnetic environment specified below. The customer or the user of the ME system
should ensure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air ±6 kV contact
±8 kV air Floors should be wood, concrete or ceramic tile. If floors are covered with
synthetic material, the relative humidity should be at least 30 %.
Electrical fast
transient/burst IEC
61000-4-4
±2 kV for power
supply lines
±1 kV for input/
output lines
±2 kV for power
supply lines
±1 kV for input/
output lines
Mains power quality should be that of a typical commercial or hospital
environment.
Surge
IEC 61000-4-5
±1 kV differential
mode
±2 kV common
mode
±1 kV differential
mode
±2 kV common
mode
Mains power quality should be that of a typical commercial or hospital
environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5 % UT
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT) for
5 cycles
70 % UT
(30 % dip in UT) for
25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
<5 % UT
(>95 % dip in UT)
for 0.5 cycle
40 % UT
(60 % dip in UT) for
5 cycles
70 % UT
(30 % dip in UT) for
25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
Mains power quality should be that of a typical commercial or hospital
environment.
If the user of the EQUIPMENT or SYSTEM requires continued operation
during power mains interruptions, it is recommended that the EQUIPMENT
or SYSTEM be powered from an uninterruptible power supply or a battery.
Power frequency
(50/60 Hz) magnet-
ic field
IEC 61000-4-8
3 A/m 3 A/m Power frequency magnetic fields should be at levels characteristic of a
typical location in a typical commercial or hospital environment.
14 Mobi Mesh / English
Declaration – electromagnetic emissions
The ME system is intended for use in the electromagnetic environment specified below.
The customer or the user of ME should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
Emissions
CISPR 14-1 Passed The ME system must emit electromagnetic energy in order to perform its intended function.
Nearby electronic equipment may be affected.
Harmonic emis-
sions
IEC 61000-3-2
Passed The ME system must be used only in a shielded location with a minimum RF shielding
effectiveness and, for each cable that exits the shielded location, a minimum RF filter
attenuation of 80 dB from 10 MHz to 20 MHz, 100 dB from 20 MHz to 80 MHz and 80 dB from 80
MHz to 100 MHz. (The minimum at 20 MHz is 100 dB and the minimum at 80 MHz is 80 dB.)
The ME system, when installed in such a shielded location, is suitable for use in all
establishments other than domestic and those directly connected to the public low-voltage
power supply network that supplies buildings used for domestic purposes.
Voltage fluctua-
tions/Flicker emis-
sions IEC 61000-3-
3
Passed

Mobi Mesh
Manual de instrucciones

16 Mobi Mesh / Español
Estimado cliente,
Este manual contiene información importante que debe conocer sobre el producto. Rogamos lo lea atentam-
ente antes del uso. Conserve siempre este manual. El caudal del nebulizador será diferente según las carac-
terísticas del medicamento; además, cuando el medicamento esté a baja temperatura, el caudal disminuirá.
USO AL QUE ESTÁ DESTINADO
Propósito - Destinatario
Este producto ha sido diseñado para inhalar medicación en caso de trastornos respiratorios. Especialistas médicos
certificados, como médicos, enfermeras y terapeutas, o personal sanitario o pacientes bajo la orientación de
especialistas médicos cualificados.
Entorno
Este producto está diseñado para ser utilizado en instalaciones médicas, como hospitales, clínicas y consultas
médicas, en la sala de una vivienda y en un entorno abierto con techado.
Garantía
El Mobi Mesh tiene una garantía limitada de dos años (excepto la boquilla, recipiente
médico, mascarilla) exento de defectos de elaboración a partir de la fecha de la compra. Cualquier pieza defectuosa
será reparada o sustituida a discreción de APEX Medical si el dispositivo no ha sufrido manipulaciones o utilizado
incorrectamente durante ese periodo. Asegurarse de que cualquier fallo no sea debido a una limpieza inadecuada o a
no haber seguido bien las instrucciones. Si fuera necesaria la reparación, póngase en contacto con el personal técnico
autorizado para recibir sus instrucciones.
NOTA-Asegúrese de conservar una copia fechada de la factura de compra para verificar que el dispositivo entra en el
periodo de garantía de 2 años.
NOTA-Esta garantía no cubre el préstamo de otro dispositivo ni la compensación de gastos de alquiler mientras dure la
reparación.

17
Mobi Mesh / Español
Dos años de vida útil de almacenamiento y seis meses de vida útil de servicio es la duración recomendada de los acceso-
rios de uso por un solo paciente. Precauciones Hay que respetar las advertencias del manual de instrucciones.
• El sistema de mascarilla no contiene DEHP.
INSTRUCCIONES IMPORTANTES DE SEGURIDAD
LEER ANTES DE UTILIZAR
Cuando se utiliza un aparato electrónico, siempre hay que tomar precauciones básicas, incluyendo las siguientes:
ATENCIÓN:
1. Es necesario aplicar un estricto control cuando este aparato se utiliza con o cerca de niños, discapacitados o
inválidos. Además, la dosis y frecuencia de uso también deben ser especificadas por un médico.
2. No hacerlo funcionar bajo la manta o almohada. Un exceso de calor podría provocar un incendio, descarga
eléctrica o lesiones a personas.
3. Utilizar este aparato solo para el uso al que está destinado según este manual.
4. No tocar la malla con bastoncillos de algodón ni con otro objeto porque el producto podría dañarse.
5. No utilizar diferentes tipos de pilas al mismo tiempo.
6. No transportar ni guardar el producto si queda todavía medicamento en el recipiente médico.
7. Seguir las instrucciones de un médico para el tipo, dosis y pauta de medicación.
8. No sumergir el producto en agua.
9. No utilizar desinfectantes que contengan hipoclorito de sodio.
10. No inhalar utilizando agua en el recipiente médico.
11. No dejar caer ni dar golpes al dispositivo principal.
12. No utilizar fijaciones ni accesorios no recomendados por el fabricante.
Other manuals for Mobi Mesh
1
This manual suits for next models
1
Table of contents
Languages:
Other Apex Digital Respiratory Product manuals