Revision status: B
File name: GA_403_EN_Plaster saw AS ELEKTRO POWER_B
Issued at: 01.03.2012
Revised at: 08.12.2022
Issued from: NS
Revised from: NS
Page 3 of 11
Instruction of use
AS Medizintechnik GmbH
Sattlerstraße 15
78532 Tuttlingen / Germany
Phone:+ 49 74 61 9 66302 6
info@AS-Medizintechnik.de EN
48-100-00 - 48-100-01
48-105-00 - 48-105-01
48-110-45 - 48-190-99
Plaster saw AS ELEKTRO POWER
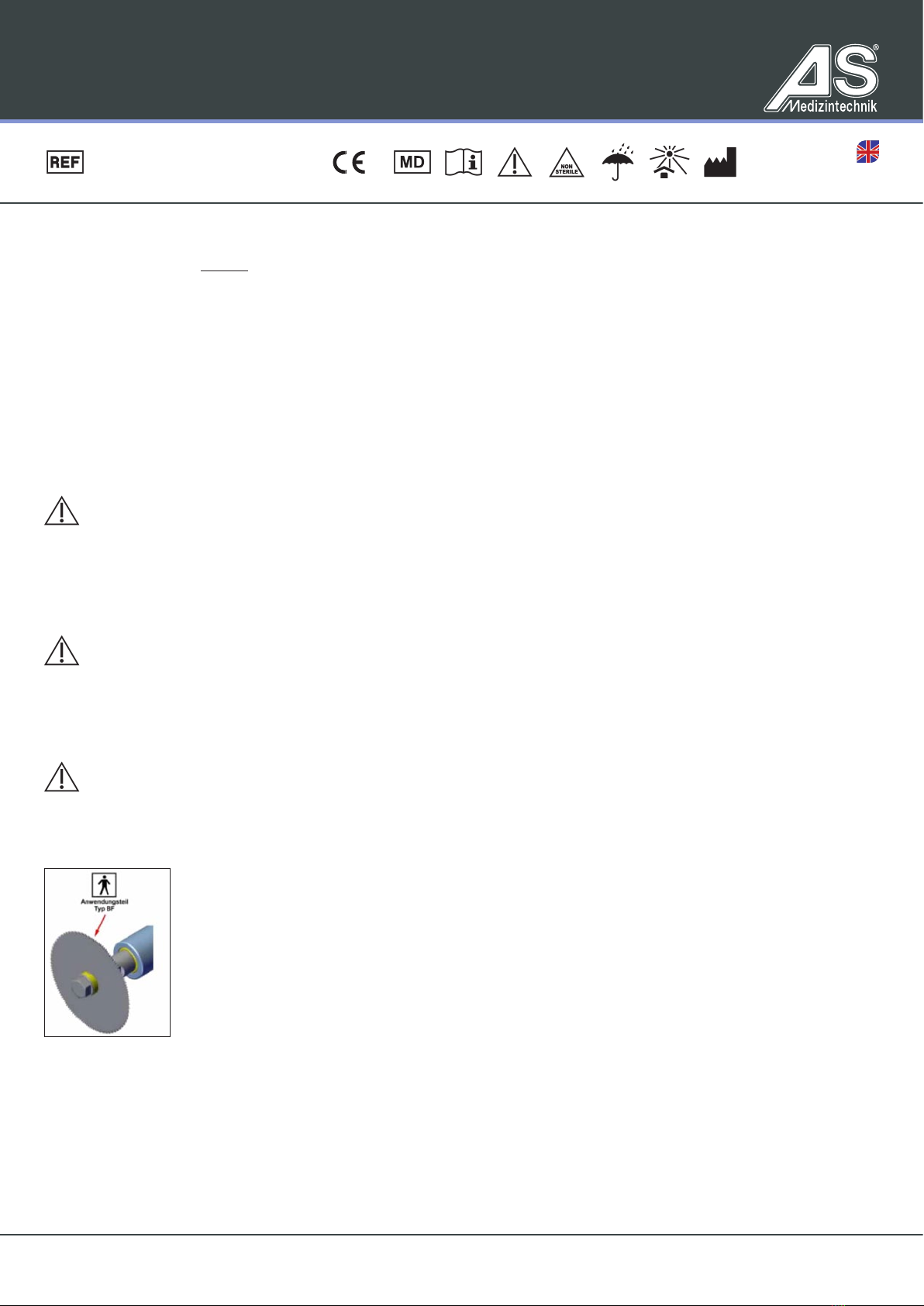
We recommend that you use the device only on a low vibration level (level 1 or 2).
Persons who have partially or fully implantable hearing systems (implantable hearing aids or similar) must switch them off or mute them if pos-
sible. The device may only be operated at a low vibration level (level 1 or 2) for such patients.
2.5 Environmental conditions
The device may only be deployed or used in rooms intended for this purpose (clinics, private practices). The electrical installations and its elec-
trical equipment must be installed at least in accordance with the applicable IEC standards and must comply with the nationally applicable laws,
regulations and requirements.
The device, the mains plug and the mains socket must not be used, switched on, operated, plugged in or used in an environment under any
circumstances,
a.) those containing oxygen,
b.) in which flammable or ignitable mixtures of anesthetics with air or oxygen or nitrous oxide or other anesthetic gases,
c.) in which flammable or ignitable mixtures,
d.) in which readily flammable or ignitable or explosive chemicals, such as skin or surface disinfectants,
regardless of its aggregate state (solid, liquid or gaseous), is enriched, can be enriched, could be enriched or is enriched.
For safety reasons, the device must never, at any time, be stored, temporarily stored or discarded in such an environment. This warning must
be strictly observed and applied at all times, without exception. Failure to do so may result in fire and/or explosion.
2.6 EMC (Electromagnetic Compatibility)
Medical electrical equipment is subject to special precautions regarding electromagnetic compatibility and must therefore be installed and
commissioned in accordance with the EMC instructions contained in the accompanying documents.
Portable and mobile RF communications equipment can affect medical electrical equipment.
The use of other mains cables and / or mains cable lengths may result in increased emission or reduced immunity of the device.
The device must not be placed directly next to or on top of other devices. If operation of the device close to or on other devices is required, the
device must be observed in order to be able to monitor its intended use / operation, in this arrangement used.
2.7 Additional information
The following additional information must be observed and complied with for the device: MPBetreibV
3 Product description
3.1 Product scope plaster saw
1 pcs. Plaster saw AS ELEKTRO POWER
1 pcs Saw blade Ø 50 mm - Item no: 48-110-50
1 pcs Saw blade Ø 65 mm - Item no: 48-110-65
1 pair Disposable key 11 mm - Item no: 48-110-50
Depending on the scope of the order, the basic equipment may vary.
Please check the consignment for damage and completeness immediately upon receipt. Later complaints can not be considered.
3.2 Electric plaster saw
The device is an oscillating saw, i.e. the saw blade does not rotate a full 360 degrees, as for example with a circular saw, but it oscillates a few degrees
clockwise and then back again. This ensures, among other things, that the sawdust is not distributed throughout the room or the entire environment.