i
®
WARNINGS
Serious Illness
• Capillary (fingerstick or Alternative Site) blood glucose
testing may not be clinically appropriate when peripheral
flow is decreased. Shock, severe hypotension,
hyperosmolar hyperglycaemia, diabetic ketoacidosis, and
occurrence of severe dehydration are examples of clinical
conditions that may adversely affect the measurement of
glucose in peripheral blood.1-3
Talk to Your Health Care Professional
• Before changing your medication based on test results,
• If your blood sugar reading is under 2.8 mmol/L , follow
medical advice immediately,
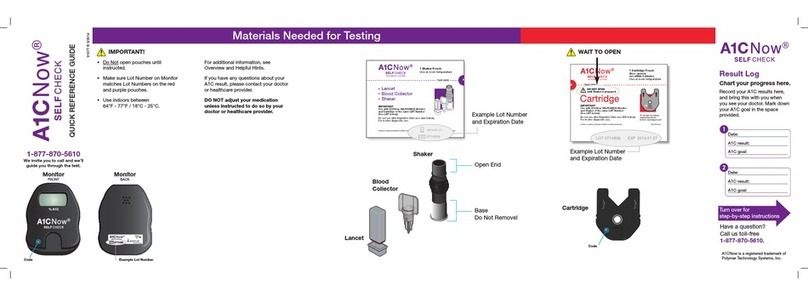
INTENDED USE
The CONTOUR®PLUS blood glucose monitoring system (meter,
test strips and control solution) is intended for self-testing by
persons with diabetes and by health care professionals to
monitor glucose concentrations in fresh capillary whole blood
drawn from the fingertip or palm, arterial and venous whole
blood or neonatal blood. It is for the quantitative measurement
of glucose in whole blood from 0.6 mmol/L to 33.3 mmol/L.
It is intended for in vitro diagnostic use only.
The CONTOUR PLUS blood glucose monitoring system may be
used as an aid to monitor the effectiveness of an individual’s
personal blood glucose control program. The CONTOUR PLUS
blood glucose monitoring system is not intended for the
diagnosis of or screening for diabetes mellitus.
IMPORTANT SAFETY INFORMATION
0006308_CntrPLUS_UG_ENuk_FpBp_v0.indd i 3/11/2020 3:32 AM
ii
• If your blood sugar reading is over 13.9 mmol/L , wash and
dry your hands well and repeat the test with a new strip. If
you get a similar reading, call your health care professional
as soon as possible,
• About whether Alternative Site Testing (AST) is appropriate
for you.
Potential Biohazard
• Always wash your hands well with soap and water and
dry them well before and after testing, handling the meter,
lancing device or test strips.
• All blood glucose measuring systems are considered
biohazardous. Health care professionals or persons
using this system on multiple patients should follow the
infection control procedure approved by their facility. All
products or objects which come in contact with human
blood, even after cleaning, should be handled as if capable
of transmitting infectious diseases. The user should
follow the recommendations for prevention of blood-
borne transmissible diseases in health care settings as
recommended for potentially infectious human specimens.
• The lancing device provided with your kit is intended for
self-testing by a single patient. It must not be used on
more than one person due to the risk of infection.
• Use a new lancet each time you test because it is no
longer sterile after use.
• Always dispose of test strips and lancets as medical waste
or as advised by your health care professional. All products
that come in contact with human blood should be handled
as if capable of transmitting infectious diseases.
• Keep out of reach of children. This kit contains small parts
which could cause suffocation if accidentally swallowed.
• Keep batteries away from children. Lithium batteries are
poisonous. If swallowed, immediately contact your poison
control centre.
0006308_CntrPLUS_UG_ENuk_FpBp_v0.indd ii 3/11/2020 3:32 AM
ii
• If your blood sugar reading is over 13.9 mmol/L , wash and
dry your hands well and repeat the test with a new strip. If
you get a similar reading, call your health care professional
as soon as possible,
• About whether Alternative Site Testing (AST) is appropriate
for you.
Potential Biohazard
• Always wash your hands well with soap and water and
dry them well before and after testing, handling the meter,
lancing device or test strips.
• All blood glucose measuring systems are considered
biohazardous. Health care professionals or persons
using this system on multiple patients should follow the
infection control procedure approved by their facility. All
products or objects which come in contact with human
blood, even after cleaning, should be handled as if capable
of transmitting infectious diseases. The user should
follow the recommendations for prevention of blood-
borne transmissible diseases in health care settings as
recommended for potentially infectious human specimens.
• The lancing device provided with your kit is intended for
self-testing by a single patient. It must not be used on
more than one person due to the risk of infection.
• Use a new lancet each time you test because it is no
longer sterile after use.
• Always dispose of test strips and lancets as medical waste
or as advised by your health care professional. All products
that come in contact with human blood should be handled
as if capable of transmitting infectious diseases.
• Keep out of reach of children. This kit contains small parts
which could cause suffocation if accidentally swallowed.
• Keep batteries away from children. Lithium batteries are
poisonous. If swallowed, immediately contact your poison
control centre.
0006308_CntrPLUS_UG_ENuk_FpBp_v0.indd ii 3/11/2020 3:32 AM
i
®
WARNINGS
Serious Illness
• Capillary (fingerstick or Alternative Site) blood glucose
testing may not be clinically appropriate when peripheral
flow is decreased. Shock, severe hypotension,
hyperosmolar hyperglycaemia, diabetic ketoacidosis, and
occurrence of severe dehydration are examples of clinical
conditions that may adversely affect the measurement of
glucose in peripheral blood.1-3
Talk to Your Health Care Professional
• Before changing your medication based on test results,
• If your blood sugar reading is under 2.8 mmol/L , follow
medical advice immediately,
INTENDED USE
The CONTOUR®PLUS blood glucose monitoring system (meter,
test strips and control solution) is intended for self-testing by
persons with diabetes and by health care professionals to
monitor glucose concentrations in fresh capillary whole blood
drawn from the fingertip or palm, arterial and venous whole
blood or neonatal blood. It is for the quantitative measurement
of glucose in whole blood from 0.6 mmol/L to 33.3 mmol/L.
It is intended for in vitro diagnostic use only.
The CONTOUR PLUS blood glucose monitoring system may be
used as an aid to monitor the effectiveness of an individual’s
personal blood glucose control program. The CONTOUR PLUS
blood glucose monitoring system is not intended for the
diagnosis of or screening for diabetes mellitus.
IMPORTANT SAFETY INFORMATION
0006308_CntrPLUS_UG_ENuk_FpBp_v0.indd i 3/11/2020 3:32 AM
90006308_CntrPLUS_UG_ENuk_FpBp_v0_placed.pdf:2