ATsens AT-Patch ATP-C70 User manual

Medical device for single use only (Reuse is prohibited) “This product is a medical device.”
The warranty period is 12 months from the date of manufacture.
AT-Patch
User Manual
MODEL: ATP-C70
UM-C-002 Ver 2.0_08/2021


AT-Patch User Manual︱3
Contents
Product Descriptions ..............................................................................................4
Components ..................................................................................................................................................4
Instructions for Use........................................................................................................................................6
CAUTIONS................................................................................................................7
Contraindications...........................................................................................................................................7
Warnings (Precautions).................................................................................................................................7
Measuring device (ATP-C70) and the App (AT-Note)...................................................................................8
S/W................................................................................................................................................................9
Conditions for Use and Storage ................................................................................................................. 10
How to Use the Device (ATP-C70)........................................................................11
Instructions for Operating and Attaching the Device (ATP-C70)................................................................ 11
Instructions for Installing the App (AT-Note) .............................................................................................. 15
Specifications.............................................................................................................................................. 17
Product Disposal...................................................................................................18
Labeling and Packaging .......................................................................................19
Labeling...................................................................................................................................................... 19
Packaging................................................................................................................................................... 20
How to Use the AT-Note App................................................................................21
Screen with the App (AT-Note) Icon & Initial Run Screen.......................................................................... 21
Connect to Device Window ........................................................................................................................ 22
Main Screen................................................................................................................................................ 23
LIVE Display Screen................................................................................................................................... 24
RECORD Screen........................................................................................................................................ 26
Symptom Note Entry Screen...................................................................................................................... 27
Symptom Note Details Screen ................................................................................................................... 29
Symptom Note Revision Screen................................................................................................................. 31
Viewing Recorded Data.............................................................................................................................. 33

4︱AT-Patch User Manual
Product Descriptions
This product (ATP-C70) is a patch-type ECG device that measures the potential
differences transmitted to the surface of the body from the action potential
generated from the activated myocardium. By attaching electrodes on certain
sites, it displays, stores, and records the measured ECG data.
Components
■Device Components
►Basic Components
AT-Patch
Pad Paper
Sealing Bag
Quick Guide
Symptom Note
Gift Box
Sub Patch
Figure 1.1 AT-Patch Basic Components
Classification: Electrocardiographic Holter Analysis
Product Name (Model Number): AT-Patch (ATP-C70)
Manufacturer: ATsens Co., Ltd.
Head Office: (13558) KINS TOWER 301, 8, Seongnam-daero 331beon-
gil, Bundang-gu, Seongnam-si, Gyeonggi-do, Republic of Korea
Factory: (13637) Point Town 803, 11, Gumi-ro, Bundang-gu,
Seongnam-si, Gyeonggi-do, Republic of Korea
Contact: Tel. 070-5220-0220/Fax. 070-8270-0738

Product Descriptions︱Components
AT-Patch User Manual︱5
►Accessories & PC S/W (AT-Report)
Accessory. Dedicated USB
cable/ATCBL-120
PC S/W(AT-Report)
USB Memory
BLE Dongle
Our dedicated cable that
connects the device with
PC S/W for the purpose of
transmitting the ECG data
recorded in the device to
PC S/W. It is connected via
the USB Port located on
the side of the device.
A USB memory stick for
storing the PC S/W
installation file to be
provided to the users. A
product with a test report or
a certificate issued by
national or accredited
offices is used.
A dedicated BLE dongle
used when connecting the
device with PC S/W
through BLE connection. A
product (BLED112) with a
test report or a certificate
issued by national or
accredited offices is used.
■App
For Android™ users: Google play™ (https://play.google.com/store)
For iPhone®users: App StoreSM (https://www.apple.com/ios/app-store/)
►Please search "AT-Note", "ATsens" in Google Play™ Store or App StoreSM.
►Download the App.
►Perform the registration step, then start using the App.
Supports: Android 5.0 or later / iOS 11.0 or later

Product Descriptions︱Instructions for Use
6︱AT-Patch User Manual
■PC S/W
►USB provided: Storage size of 4 GB or greater & a CE-certified product
►Minimum requirements (PC)
Feature
Specification
Processor
Intel Core i7-9700K
RAM
16 gigabyte (GB)
Hard disk space
Main SSD: 512GB/Back-up HDD: 1TB
Graphics card
DirectX 9 or later with WDDM 1.0 driver
Display
1920 x 1080 / 24 inch Full-HD Monitor
OS
Windows 10 (64bit)
Instructions for Use
►ATP-C70 is a device that measures, records, and stores the data from one ECG channel, and
transmits the measured data using wireless communication (Bluetooth) to be displayed on the App
and the PC S/W.
►Data to be recorded
ECG Signal
3-axis Data
Heart rate

AT-Patch User Manual︱7
CAUTIONS
This product is to be used by the patient themself; the intended operator is the patient.
Contraindications
►DO NOT use if you have current symptoms or medical history of skin cancer, rash, dermatosis, keloids,
wounds, etc.
Warnings (Precautions)
►This product is for single use only. Reuse is prohibited. Reusing it may lead to malfunction and
inaccurate results.
►DO NOT attach to any other place than the recommended bodily location of application.
►Only authorized technicians are allowed to repair or disassemble ATP-C70.
►Make sure to be fully aware of how to use the product, through sufficient training, before using it.
►Avoid use at locations where wireless communication interference may occur (e.g., places where metallic
hardware or electronic devices are frequently found).
►This product cannot be simultaneously used with a defibrillator.
►DO NOT expose to strong electromagnetic fields.
►Product disposal
Disposal of ATP-C70 and battery must comply with local waste disposal regulations. Non-compliance
with waste disposal regulations may result in environmental pollution.
NOTE: The data stored within the device must be taken care of before disposal.

CAUTIONS︱Measuring device (ATP-C70) and the App (AT-Note)
8︱AT-Patch User Manual
Measuring device (ATP-C70) and the App (AT-Note)
■Caution
►Since improper application and use of the sensor may result in inaccurate measurements, please
avoid:
Excessive movement on the patient's part
Application outside the recommended bodily locations
To prevent signal abnormalities due to the state of the patient's skin, sufficient notice of use from
a medical specialist must be obtained before use.
►The following persons should consult a physician before using the device.
Patients with sensitive skin or skin allergy
Patients with wounds on the skin that contact the device
Pregnant women, breastfeeding mothers, infants or children
Patients with pacemakers, defibrillators, or other implantable electronic devices
►Take caution to avoid getting liquid in the device, and also avoid the following:
(Dustproof and waterproof grade IP44)
Long-term exposure to water such as baths, swimming, etc.
►Avoid letting the device come into contact with organic compounds like thinners or benzene.
►Beware of strong shocks and vibrations.
►Once the product is detached from the body, do not reattach it.
►The smartphone on which the App is run must be one with a test report or a certificate issued by
national or accredited offices.

CAUTIONS︱S/W
AT-Patch User Manual︱9
S/W
►This Holter ECG recorder is intended to provide diagnostic aid.
►It may be used within the boundaries of medical laws.
►This Holter ECG recorder and installed equipment require a Bluetooth connection.
The product functions by connecting a single device with a single software (App, PC S/W);
connecting multiple devices is not supported.
►Cautions when handling patient's personal information
Protection of patient's personal information and compliance with the [Privacy Act] is of great
importance to ATsens.
Patient's personal information will not be used for any purpose outside analyzing the patient's cardiac
impulse and data management; in the case where the purpose of use is changed, consent must be
obtained beforehand.
Personal information collected from patients is stored and managed for up to 5 years.
Personal information that elapses the retention period or that is no longer required to fulfill the
identified purposes will be destroyed immediately.
The following measures are being taken to ensure the safety of information.
- Administrative Measures: Annual personal information protection act training
- Technical Measures: Encryption of personal information, installing security software, managing
access to personal information data, and storing access records for more than 6 months
- Physical Measures: Restricting unauthorized access and using document encryption device.
Our dedicated cable must be used when transmitting the ATP-C70's ECG data.
- ECG data cannot be transmitted using commercial USB cables.
The PC on which the S/W is used must meet following requirements.
- A product with a test report or a certificate issued by national or accredited offices must be used.
- A product protected by an anti-virus software must be used.
This S/W does not utilize any network connection.
- No network connection, such as a closed network within a medical facility, shared network, etc., is
used; data is stored within the PC on which the S/W is installed. (No external transmission)
Measures to be taken when a cybersecurity issue occurs.
- The manufacturer should be consulted if a cybersecurity issue occurs.
- ATsens Co., Ltd.: Tel. 070 5220 0738, Fax. 070 8270 0738, E-mail. sales@atsens.com

CAUTIONS︱Conditions for Use and Storage
10︱AT-Patch User Manual
Conditions for Use and Storage
■Conditions for Use
►Temperature range: 10℃~ 45℃
►Relative humidity range: 10% ~ 95%, non-condensing
►Atmospheric range: 700hPa ~ 1060hPa
►Dustproof and waterproof grade: IP44
■Conditions for Storage
►Temperature range: -20℃~ 55℃
►Relative humidity range: 0% ~ 95%, non-condensing
►Atmospheric range: 700hPa ~ 1060hPa

AT-Patch User Manual︱11
How to Use the Device (ATP-C70)
Instructions for Operating and Attaching the Device (ATP-C70)
Figure 1.2 External appearance of the device
No.
Name
Description
1
Case Top
The Case Top is made of polycarbonate material, with the ATSens
logo and S/N of the device printed on the surface.
2
Case BTM
Case Bottom is made of polycarbonate material and contains ECG
electrodes.
3
Power Button/LED
The Power Button turns the power ON, and the power status can
be confirmed through LED.
4
Main Patch
Main Patch attaches to the surface of the skin. Medical grade
adhesive tape is used.
5
Sub Patch
Sub Patch allows firmer attachment. Medical grade adhesive tape
is used.
6
Hydrogel
Hydrogel, positioned between the ECG electrodes and the skin,
allows for the measurement of ECG signals under uniform
conditions.
7
Electrodes
ECG electrode
8
Battery
Coin Battery / CR2032
9
USB Port
Dedicated USB cable can be connected through the USB Port after
removing the USB port cap.
Table 1.1 Table describing the external appearance of the device (ATP-C70)

How to Use the Device (ATP-C70)︱Instructions for Operating and Attaching the Device (ATP-C70)
12︱AT-Patch User Manual
■Instructions for Operating the Device (ATP-C70)
①The device (ATP-C70) is initially packaged in Sleep Mode and delivered to the customer. Patient
who wishes to attach the device should press the Power Button, #❸in [Figure 1.2], for 3 seconds,
upon which the green LED blinks twice and a long “beep” sound occurs once. The device is now in
Active Mode.
②Once the device (ATP-C70) is attached and connected to the App (AT-Note), it begins storing
measured ECG signals in the internal memory. The device continues to store the ECG signals for
the duration of use, and automatically goes into Sleep Mode after use. NOTE: If there is no App or
AT-Report (PC S/W) Hookup connection for about 8 minutes after Active Mode is on, then the
device goes into Sleep Mode following a single red LED blink and three “beep-- bebeep--” sounds.
•During the initial connection process, the connection between the device (ATP-C70) and AT-Report
(PC S/W) Hookup or the App must be established at least once. Otherwise, the connection process
is terminated as described in ②, and the device will not operate normally. After the initial
connection is established, ECG measurement is performed normally even if AT-Report (PC S/W)
Hookup or the Bluetooth paring for the App is terminated for some reasons.
- The device cannot be used with a PC or smartphone that does not support Bluetooth.
•The working status of the device (ATP-C70) can only be confirmed through the App's real-time live
monitoring or AT-Report (PC S/W) Hookup.
•Once the initial one-time connection is confirmed, AT-Patch is not terminated for the duration of use
(7 days).
- Physically turning the power off is not possible. Automatic Power-Off will occur after the duration
of use (7 days) has elapsed.
- In case the buzzer or the Red LED blink was missed after AT-Patch's duration of use has
passed, the App or AT-Report (PC S/W) Hookup can be used for confirmation.

How to Use the Device (ATP-C70)︱Instructions for Operating and Attaching the Device (ATP-C70)
AT-Patch User Manual︱13
■Instructions for Attaching the Device on the Body (ATP-C70)
►State of the skin before attachment
An area larger than the device (ATP-C70) attachment area needs to be cleaned on the patient's skin.
If body hair is abundant in the attachment area, shaving will be required to remove the hair.
NOTE: If a wound or bleeding occurs when removing body hair, the device should be attached after
the bleeding stops.
As demonstrated in the "confirm patch
attachment site" figure ❶, place the end of
the patch about a finger's distance below the
clavicle, and confirm the attachment site at a
45-degree angle.
If necessary, remove possible obstructions like
hair using a razor, etc. as shown in the "shave
the patch attachment site" figure ❷.
Figure 1.3 Attachment preparation step –Confirming attachment site
To produce the accurate ECG signals, clean the attachment area using alcohol swabs to remove any
possible obstructions such as dead skin cells, etc. from the skin surface. Then, fully dry the skin for at
least one minute before attaching the device (ATP-C70).
Cleansing the patch attachment site with alcohol
As illustrated in Figure ❸, cleanse the attachment site to remove dead skin cells and possible
obstructions by wiping horizontally, vertically and diagonally with alcohol cottons or swabs. Then,
fully dry for at least one minute to make sure no alcohol is left on the surface.
Figure 1.4 Attachment preparation step –Removing possible obstructions from the attachment site

How to Use the Device (ATP-C70)︱Instructions for Operating and Attaching the Device (ATP-C70)
14︱AT-Patch User Manual
►Attaching the device to the body
After pressing the device (ATP-C70) Power Button, attach as follows
Figure 1.5 How to attach ATP-C70
1. As shown in [Figure 1.5] ❶, remove the tape designed to protect the patch side. Once the
protective tape is removed, attach the device to the body.
2. As shown in [Figure 1.5] ❷, attach the device so that it is tilted toward the left nipple area, using
the line in the middle between the two clavicles as the center. However, if the patient has a fuller
chest or if diagonally attaching the device is difficult, the part directed toward the nipple should be
raised a bit higher and then attached.
3. As shown in [Figure 1.5] ❸, remove the bottom protective tape from the ECG Sub Patch, then
attach it in line with the Main Patch. Apply even pressure on the attached Sub Patch.
4. As shown in [Figure 1.5] ❹, evenly apply pressure on the patch so that no gap occurs
when the device is attached to the body, and then remove the protective tape.
How to Use the Device (ATP-C70)︱Instructions for Installing the App (AT-Note)
AT-Patch User Manual︱15
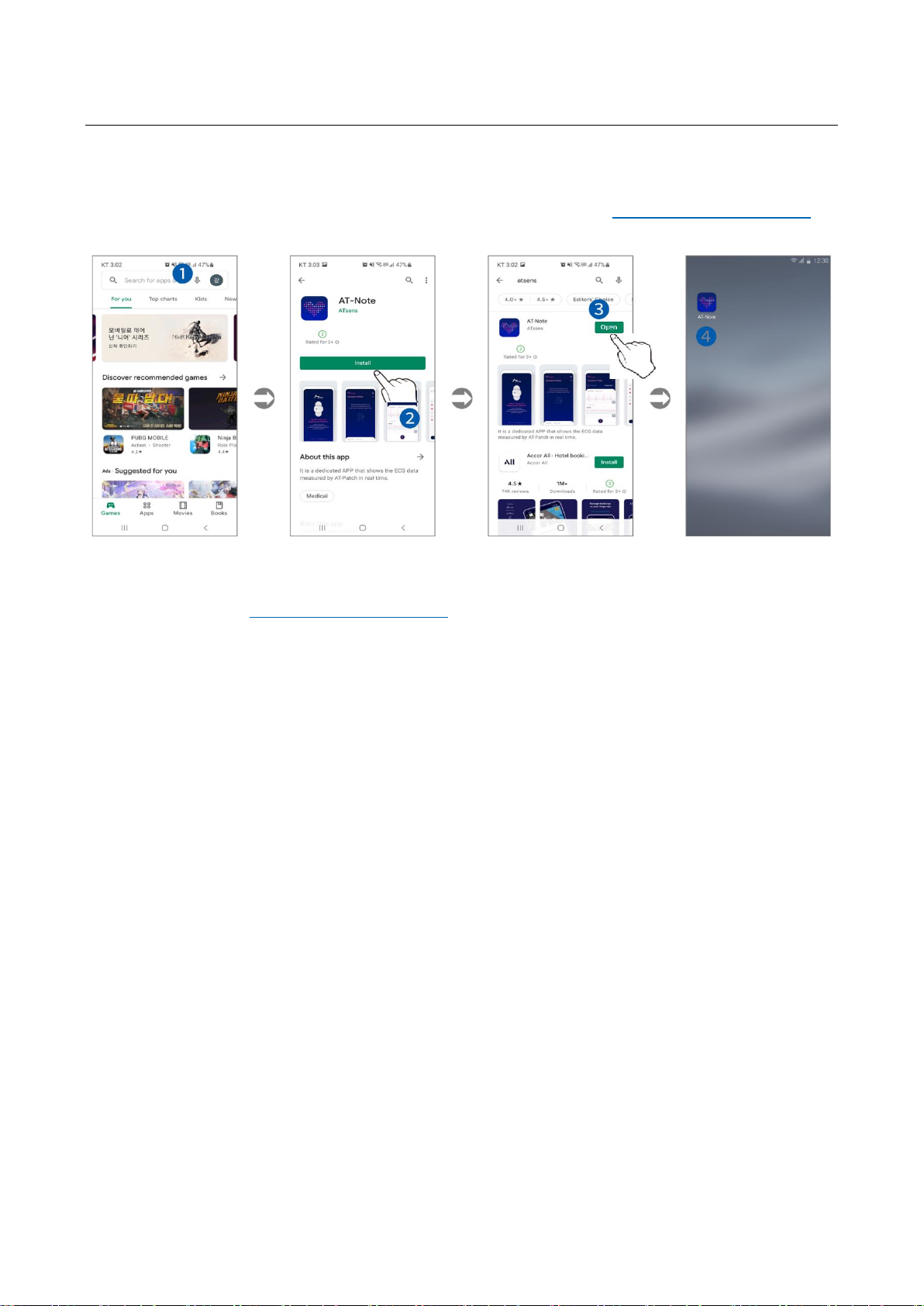
Instructions for Installing the App (AT-Note)
►Android™ users can download App (AT-Note) from Google play™ (https://play.google.com/store)
on Android™ Market. [Figure 1.6] demonstrates the installation steps.
Figure 1.6 Installing the App –Android
1. As shown in [Figure 1.6] ❶, search for either AT-Note, ATsens, AT-Patch in the Android™ market
Google Play™ (https://play.google.com/store) search field.
2. As shown in [Figure 1.6] ❷, click Install to download and install the App once the search result has
returned AT-Note.
3. As shown in [Figure 1.6] ❸, click Open to run the App when the installation is completed.
4. Once the AT-Note icon appears on the smartphone screen as shown in [Figure 1.6] ❹, the
installation has been successfully completed.
5. After installation, use according to How to Use the AT-Note App.

How to Use the Device (ATP-C70)︱Instructions for Installing the App (AT-Note)
16︱AT-Patch User Manual
►For iPhone users (iPhone®), App (AT-Note) can be downloaded from App StoreSM
(https://www.apple.com/ios/app-store/) in the iOS Store. [Figure 1.7] demonstrates the installation
steps.
Figure 1.7 Installing the App –iOS
1. As shown in [Figure 1.7] ❶, search either AT-Note or ATsens in the iOS App StoreSM
(https://www.apple.com/ios/app-store/) search field.
2. As shown in [Figure 1.7] ❷, click Install to download and install the App once the search result has
returned AT-Note.
3. As shown in [Figure 1.7] ❸, click Open to run the App when the installation is completed.
4. Once the AT-Note icon appears on the smartphone screen as shown in [Figure 1.7] ❹, the
installation has been successfully completed.
5. After installation, use according to How to Use the AT-Note App.
How to Use the Device (ATP-C70)︱Specifications
AT-Patch User Manual︱17
Specifications
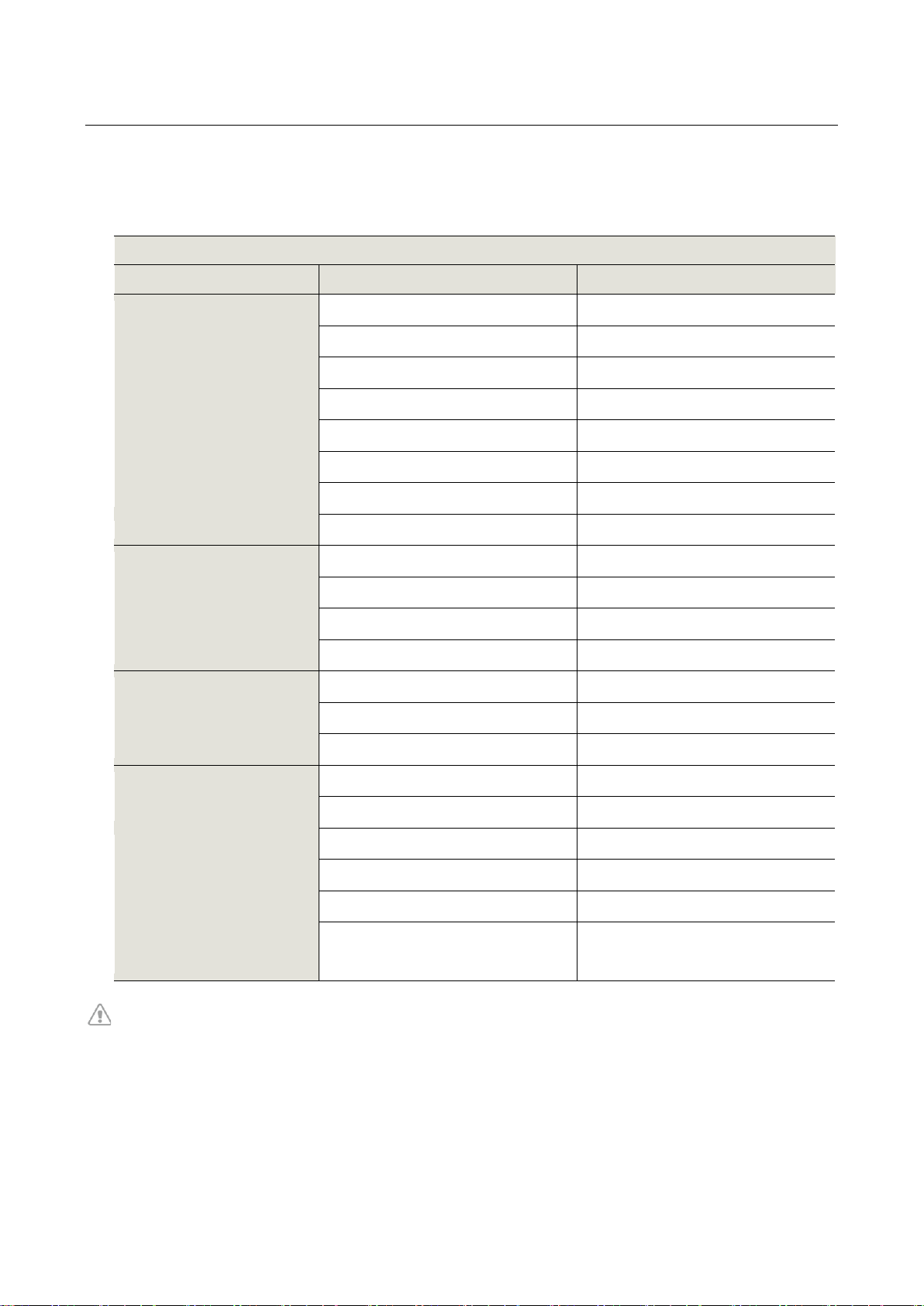
■ATP-C70 Specifications
ATP-C70
Item
Description
ECG
Type
BF type
Sampling Rate
250 sample/sec
Input Offset Dynamic Range
±300mV
Channel
1 channel
ADC Resolution
10 bits
Input Impedance
≥10MΩ
Frequency Response
0.05Hz to 40Hz
Electrode
AC impedance
Less than 3KΩ (10Hz)
RF
RF communication
2.4GHz BLE 4.2
Effective Radiated Power
<1mW
RF Frequency Band of TX
2.4GHz
Bandwidth of the Receiver
2400 ~ 2480MHz
S/W
CPU
ARM Cortex-M4
Supported App
Up to Android 5.x or iOS 11.x
Supported PC S/W Version
Window 10 (64bit)
Power Requirement
Power Supply
DC 3V, Coin Battery (CR2032)
Battery Life
Up to 7 days
Physical
Characteristics
Total Size (L x W x H: mm)
74 x 47.05 x 8.4
Main Body Size (L x W x H: mm)
32.6 x 39.6 x 7.7
Sub Patch Size (L x W x H: mm)
95 x 68.05 x 0.05
Weight (g)
Main Body: Below 12g
Sub Patch: Below 1g
App (AT-Note) / PC S/W (AT-Report): The smartphone and PC used must be one with a test
report or a certificate issued by national or accredited offices.

18︱AT-Patch User Manual
Product Disposal
►This product is a medical device for single use only. Reuse is prohibited.
►Disposal of ATP-C70 and battery must comply with local waste disposal regulations. Non-
compliance with waste disposal regulations may result in environmental pollution.

AT-Patch User Manual︱19
Labeling and Packaging
Labeling
■Package Label
■Explanation of Visual Symbols of Label
No.
Symbol
Descriptions
1
The serial number that identifies the object
2
Date of manufacture
3
For European Authorized Representative
4
Keep dry
5
Protected against solid objects bigger than 1mm / Water splashing from all
directions.
6
Caution
The equipment may be damaged if the instruction is not observed
7
Instruction for User manual
8
Do Not Reuse (Disposable medical devices)
9
Type of applied part
10
Manufacturer
11
CE Marking of Conformity

Labeling and Packaging︱Packaging
20︱AT-Patch User Manual
Packaging
■Basic Components
Classification
Internal
packaging
Components
Quantity
Packaging
Material
Tools Used
Basic
Components
External: Box gift
Bag Sealing Gift
1EA
Paper
Scissors / Tape
Bag Sealing Gift
ATP-C70
1EA
BAG GIFT
Scissors
Sub Patch
1EA
-
Handling
Symptom Note
1EA
Vellum paper
Handling
Quick Guide
1EA
Vellum paper
Handling
■PC S/W & Accessories
Classification
Internal
packaging
Components
Quantity
Packaging
Material
Tools Used
Accessories
Dedicated USB Cable
2EA
BAG PE
Handling
USB Memory
PC S/W(AT-Report)
1EA
BAG PE
Handling
BLE Dongle
BLE Dongle
1EA
BAG PE
Handling
Table of contents
Other ATsens Personal Care Product manuals