Belmint BEL-TENS User manual

TENS MASSAGER
TENS
MASSAGER
www.belmint.com
@belmintsproducts
@belmintproducts

Congartulations on your purchase!
Your Belmint Tens Unit Muscle Stimulator Electronic Pulse
Massager enables you to control the amount of massage
pressure your tired and sore muscles recieve.
Pain relief is just minutes away with the Belmint
Transcutaneous Electrical Nerve Stimulator. Gentle
electrical pulses provide powerful relief to sore and
aching muscles, joints and nerves from overuse, injury,
and chronic problems like arthritis. Reusable adhesive
pads let you precisely treat the source of the pain.
Electrical pulses target the central nervous system,
providing relief without conventional medicines.
Portable & lightweight, you can use the Belmint system
at home, in the offce,or while away on travel. Stores
easily in luggage, briefcase, or handbag. With 5 massage
modes and 16 intensity levels, it is easy to customize the
stimulator for perfect relief with every use. Safe for daily
use.
Developed by Belmint engineers using high quality,
durable materials,your massager is easy to clean and
made to last.
To celebrating your comfort,
The Belmint Team
Read All Instructions Before Using:
BEL-TENS Electronic Pulse Massager is for over the counter use and
for home use, which delivers electric pulses to tired and sore
muscles. These pulses are generated by the device and delivered
through electrodes on the skin to trigger contraction of the muscles.
BEL-TENS is portable, compact, and stylish in design. The BEL-TENS
device has five auto operational programs represented by five
selection buttons of body areas through two channels with four
attachment pads and shows graphic information about mode style,
intensity and time remaining on a LCD based display which is
incorporated within the device body.
Indications For Use:
To be used for temporary relief of pain associated with sore and
aching muscles in the shoulder, waist, back, arm, and leg, due to
strain from exercise or normal household and work activities
What’s In The Box?
1 x Tens Unit Massager Device
4 x Electrode Pads
2 x Electronic Lead Wires
4 x AAA Batteries
1 x User Manual Guide
Introduction
1 2
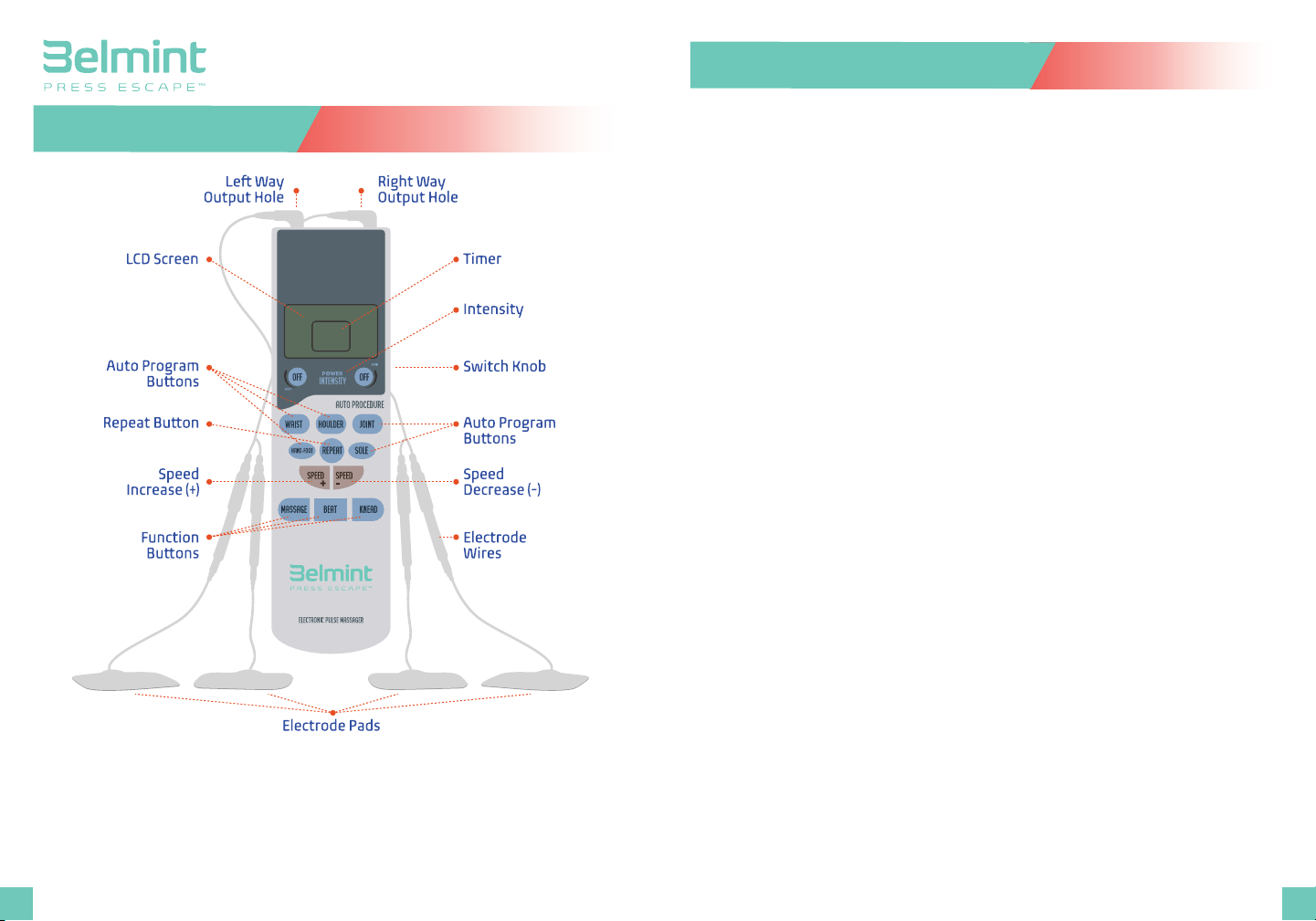
Operation
1. Install 4 AAA Batteries into the device. Match the positive
electrode and negative electrode in the right direction;
2. Connect 1 pair of electrode wire into the upper left hole of the
device and another pair of electrode wire in the upper right hole.
3. Attach the electrode pads (connecting to the electrode wire) to
the treatment area, such as shoulder.
4. Turn the power/intensity switch knob to 1. The screen should
show P. This means the device is ready to begin treatment.
5. Press one of the function keys to start treatment, such as shoulder
or massage.
6. Press SPEED+ or SPEED- to adjust the speed; Rotate the Switch
Knob to adjust the intensity.
NOTE:
Start from the lowest speed and intensity, and then gradually adjust
to a comfortable level on a scale level from to 1 to 10.
Recommended Practices
1. Duration is 10 – 15 Minutes for each treatment area.
2. Frequency is 2 Times per day per area.
3. Intensity and speed selections are based upon the level of your
comfort
Cleaning & Maintenance
Please use water or neutral detergent to clean the device first then
using the dry cloth to wipe it again. The electrode pads coming with
the device are disposable, and should be replaced when their
adhesiveness becomes worse. Contact the seller for replacements.
Do not let the sticky side of the pad touch anything, including greasy
finger tips.
Caution!
All servicing of this massager MUST be performed by authorized
BELMINT personnel ONLY.
Note:
The Tens Massager Package will automatically Shut OFF after 15
Minutes for your saftey.
Product Details
Massager Function
34
READ ALL INSTRUCTIONS BEFORE USING:
DANGER
Do not use this device if you have an implanted defibrillator or
implanted metallic devices. Such use could cause electrical shock,
burns, electrical interference or death.
WARNING
If you have one of the following conditions, please consult with your
physician before purchasing or using this device.
Acute disease, malignant tumor, infective disease, pregnant, heart
disease, high fever, abnormal blood pressure, lack of skin sensation
or an abnormal skin condition, any condition requiring the active
supervision of a physician.
NOTICE
- DO NOT use this device while driving, sleeping, or in high humidity
areas: such as bathroom.
- Keep the device away from wet, high temperature and
direct-sunlight place.
- Keep this device out of reach of children.
- Stop using this device at once if you feel pain, discomfort, dizziness
or nausea and
consult your physician.
- DO NOT attempt to move the electrode pads while the device is
operating.
- DO NOT use the device around the heart, on the head, mouth,
pudendum or blemished skin areas.
DO NOT APPLY STIMULATION OF THIS DEVICE IN THE FOLLOWING
CONDITIONS:
1) Across the chest because the introduction of electrical current
into chest may cause rhythm disturbances to the heart, which could
BE AWARE OF THE FOLLOWING:
1) Consult with your physician before using this device. The
simulation with the device may: 1. Cause lethal rhythm disturbances
to the heart in susceptible individuals, and, 2. Disrupt the healing
process after a recent surgical procedure;
2) Device is not effective for pain of central origin, including
headache;
3) Device is not substitute for pain medications and other pain
management therapies;
4) That the device has no curative value;
5) Device is a symphomatic treatment and, as such, suppresses the
sensation of pain that would otherwise serve as a protective
mechanism;
6) User may experience skin irritation, burns or hypersensitivity due
to the electrical stimualtion or electrical conductive medium (gel);
7) If user has suspected or diagnosed epilepsy, follow precautions
recommended by his or her physician;
8) Use caution if stimulation is applied over the menstruating uterus;
Saey Warning
Features
be lethal;
2) Over painful areas. Please consult with your physician before using
this device if you have painful areas;
3) Over open wounds or rashes, or over swollen, red, infected, or
inflamed areas or skin eruptions (e.g., phlebitis, thrombophlebitis,
varicose veins). Apply stimulation only to normal, intact, clean,
healthy skin;
4) In the presence of electronic monitoring equipment (e.g., cardiac
monitors, ECG alarms). The electronic Stimulator may not operate
properly when the electrical stimulation device is in use;
5) While operating machinery, or during any activity in which
electrical stimulation can put you at risk of injury; on children
9) Use caution if stimulation is applied over areas of skin that lack
normal sensation;
10) Use this device only with the leads, electrodes, and accessories
that the manufacturer recommends.
Medical Electrical Equipment needs special precautions regarding
Electromagnetic Compatibility (EMC) and needs to be installed and
put into service according to the EMC information provided.
Portable and Mobile Radio Frequency (RF) communication
equipment can effect Medical Electrical Equipment.
56
ENVIRONMENTAL CONDITION FOR TRANSPORT AND STORAGE:
Easily fragile product.
Keep the product in the dry place away from water and rain.
Product package should be recycled.
Stacked up 4 cartons at most.
Temperature Range: 0°c to 48°c
Humidity: 10% to 85%
Atmospheric Pressure: 400hPa to 1060hPa
SETUP:
Unpack the box of the product, take the product and batteries out,
place the batteries into the device at its back compartment, and
connect the electrode pads into the device through the wires.
BE AWARE OF THE FOLLOWING:
1) Consult with your physician before using this device. The
simulation with the device may: 1. Cause lethal rhythm disturbances
to the heart in susceptible individuals, and, 2. Disrupt the healing
process after a recent surgical procedure;
2) Device is not effective for pain of central origin, including
headache;
3) Device is not substitute for pain medications and other pain
management therapies;
4) That the device has no curative value;
5) Device is a symphomatic treatment and, as such, suppresses the
sensation of pain that would otherwise serve as a protective
mechanism;
6) User may experience skin irritation, burns or hypersensitivity due
to the electrical stimualtion or electrical conductive medium (gel);
7) If user has suspected or diagnosed epilepsy, follow precautions
recommended by his or her physician;
8) Use caution if stimulation is applied over the menstruating uterus;
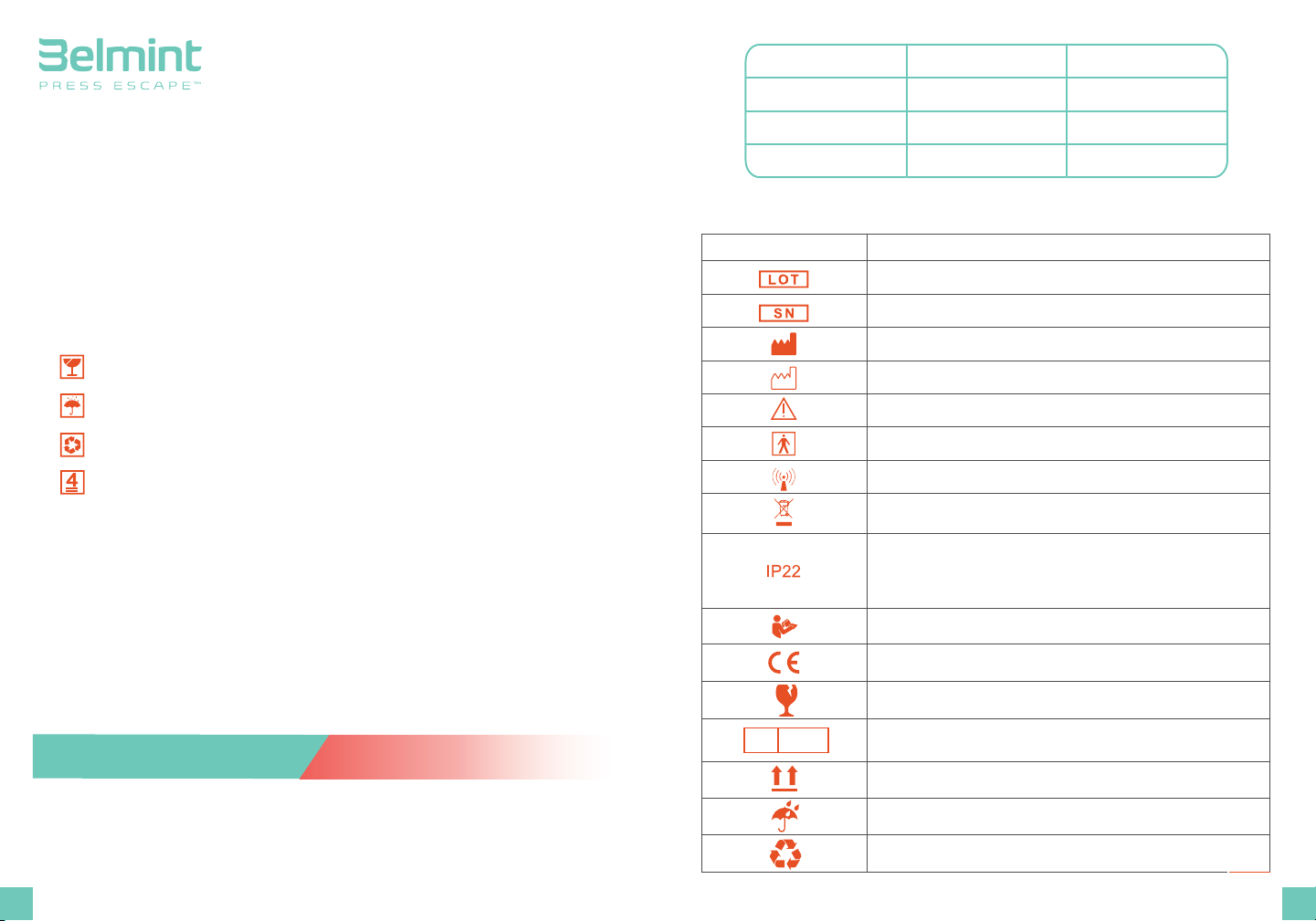
SYMBOLS:
Graphic symbol Meaning
Manufacturer
Date of Manufacturer
Caution
Type BF applied part
Low-frequency electromagnetic radiation
"WEEE (Waste Electrical and Electronic Equipment)". The
waste products should be handled legally.
Dustproof waterproof level. It can prevent solid object
larger than 12mm from intruding, and when tilt for 15
degrees, it can still prevent water from intruding, so no
harmful effect will be created.
Follow instructions for use
Fragile, handle with care
Symbol for "AUTHORISED REPRESENTATIVE IN THE
EUROPEAN COMMUNITY"
This way up
Keep the product in the dry place Away from water and rain.
Product package should be recycled.
CE mark
Batch code
Serial number
1639
EC REP
Program
Mode 1
Mode 2
Mode 3
Pulse Rate (Hz) Pulse Width (μs)
62.5 100 ~ 240
500
100 ~ 240
1
62.5
9) Use caution if stimulation is applied over areas of skin that lack
normal sensation;
10) Use this device only with the leads, electrodes, and accessories
that the manufacturer recommends.
Medical Electrical Equipment needs special precautions regarding
Electromagnetic Compatibility (EMC) and needs to be installed and
put into service according to the EMC information provided.
Portable and Mobile Radio Frequency (RF) communication
equipment can effect Medical Electrical Equipment.
Rated Voltage
DC 6V
Model
BEL-TENS
Rated Frequency
1-62.5Hz
Technical Details
Rated Power
0.48 W
78
Safety Standards
Medical Devices Directive 93/42/EEC
IEC 60601-1:2005+A1:2012/EN 60601-1:2006 Medical electrical
equipment - Part 1: General requirements for basic safety and
essential performance.
IEC 60601-1-2:2007/EN 60601-1-2:2007 Medical electrical equipment
- Part 1-2: General requirements for safety - Collateral standard:
Electromagnetic compatibility - Requirements and tests.
IEC 60601-2-10:2012/EN 60601-2-10:2000+A1:2001 Medical electrical
equipment - Part 2-10: Particular requirements for the safety of nerve
and muscle stimulators.
IEC 60601-1-11:2010 Medical electrical equipment -- Part 1-11: General
requirements for basic safety and essential performance -- Collateral
standard: Requirements for medical electrical equipment and
medical electrical systems used in the home healthcare
environment.
EN 980 Symbols for use in the labeling of medical devices.
EN 1041 Information supplied by the manufacturer with medical
devices
IEC/60601-1-6/ EN 60601-1-6 Medical electrical equipment – Part1-6:
General requirements for basic safety and essential performance –
Collateral standard: Usability.
IEC 60601-1-11/ EN 60601-1-11 Medical electrical equipment – Part
1-11: General requirements for basic safety and essential
IEC 60601-1-11/ EN 60601-1-11 Medical electrical equipment – Part
1-11: General requirements for basic safety and essential
performance – Collateral standard: Requirements for medical
electrical equipment and medical electrical systems used in home
The subject device has been tested and found to comply with the
limits for a Class B digital device, pursuant to part 15 of the FCC
rules. These limits are designed to provide reasonable protection
against harmful interference in a residential installation.
The product generates, uses, and can radiate radio frequency
energy and, if not installed and used accordance with the
instructions, may cause harmful interference to radio
communications.
However, there is no guarantee that the interference will not occur
in a particular installation. If the product does cause harmful
interference to radio or television reception, which can be
determined by turning the product on or off, the user is encouraged
to try to correct the interference by one or more of the following
measures:
a.) Reorient or relocate the recieving antenna;
b.) Increase the separation between the product and the receiver;
c.) Consult the dealer or an experienced radio/TV technician for
help.
d.) Connect the equipment into an outlet on a circuit different from
that to which the receiver is connected.
Changes or modifications to this product not expressly approved by
the party responsible for compliance could void the user’s authority
to operate the equipment.
healthcare environment.
IEC 62304/ EN 62304 Medical device soſtware - Soſtware life-cycle
processes.
IEC 62366/ EN 62366 Medical devices – Application of usability
engineering to medical devices.
ISO 10993-1 Biological evaluation of medical devices - Part 1:
Evaluation and testing within a risk management process.
FCC COMPLIANCE STATEMENT:
910

MODEL TYPE
POWER SUPPLY
PL-009
4 x AAA Batteries
Waveform and
Wave Shape
Pulse Duration
Pulse Frequency
Output Voltage
Treatment Time
Output Intensity
Modes
Single Wave Pulse
100 - 500us (Microseconds)
1-62.5Hz
(Hz=vibration per second)
Max. 30Vpp ± 20%
(at 500ohm load)
15 Minutes
0 to 10 Levels, Adjustable
3 Auto Modes
15 Minutes
Type BF applied part
Internally Powered
Equipment
(Not Applicable)
IP22
1 Year
Storage for 2 Years (No Use)
Times of Reusable: 30 Times
Continuous Operation
WEIGHT
AUTO SHUTOFF
Degree of Protection
Against Electric Shock
Type of Protection
Against Electric Shock
Grade of Waterproof
Product Life
Lifetime for Electrode
Mode of Operation
AO
Soware Version
Note: Not intended to be sterilized.
Not for use in an OXYGEN RICH ENVIRONMENT
137g
11 12
Table of contents
Other Belmint Massager manuals

Belmint
Belmint BEL-BNM User manual

Belmint
Belmint BEL-BNM User manual

Belmint
Belmint BEL-CHM User manual

Belmint
Belmint BELFOOTMAS User manual

Belmint
Belmint BEL-CBC User manual

Belmint
Belmint BEL-SEAT User manual

Belmint
Belmint BEL-FBM User manual

Belmint
Belmint BEL-SMM User manual

Belmint
Belmint BEL-TMP User manual

Belmint
Belmint BEL-HHM User manual
Popular Massager manuals by other brands

Lifepro
Lifepro Recovery + Fitness VIBRACARE user manual

Silvercrest
Silvercrest SSNR 12 A1 Operation and safety notes

SereneLife
SereneLife SLNKMSG131 user manual

Silvercrest
Silvercrest SSMK 40 B2 Operation and safety notes

Pro-Form
Pro-Form Restoration 831.270000 user manual
Core Nine
Core Nine 8825 quick start guide