Beta Bionics iLet Bionic Pancreas System User manual

LA000039 J
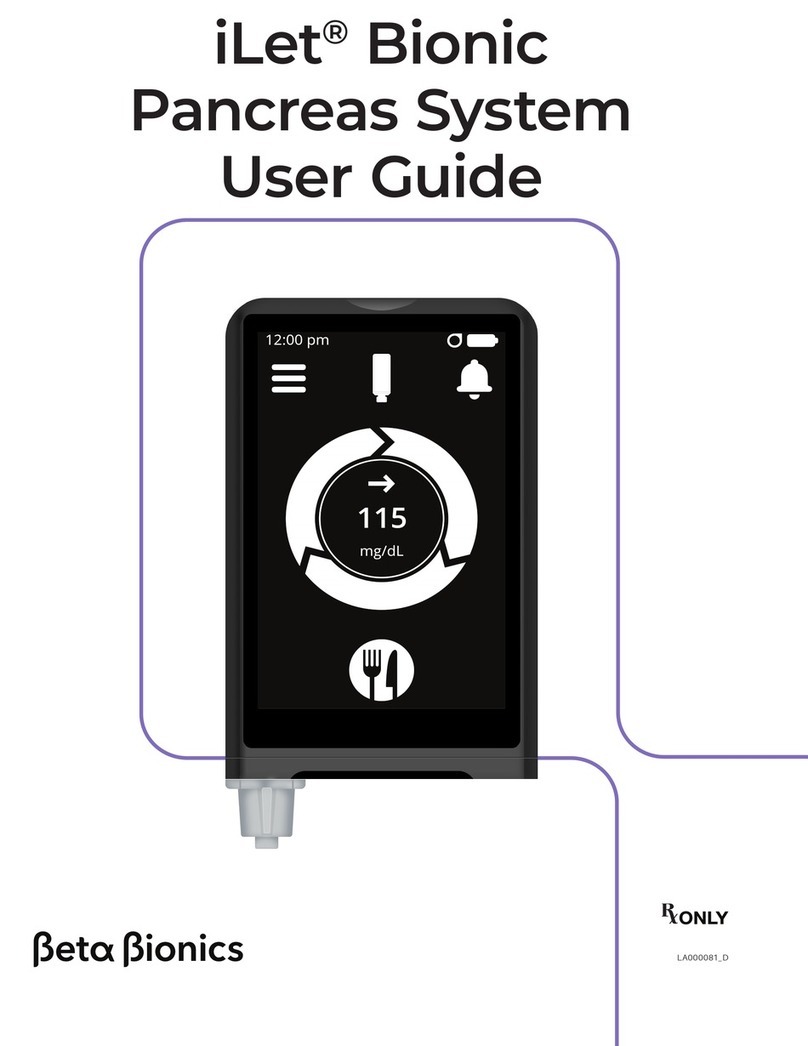
iLet®Bionic
Pancreas System
User Guide

II IIIiLet Bionic Pancreas System User Guide | iLet Bionic Pancreas System User Guide |
Manufacturer
Beta Bionics, Inc.
11 Hughes
Irvine, CA 92618
USA
Customer Service
Tel +1-855-745-3800
Email support@betabionics.com
Equipment covered in this User Guide
iLet® Bionic Pancreas
iLet Cartridge
iLet Connect
iLet Charge
The information, text and/or images within this document, or any portion thereof, may not
be copied, displayed, downloaded, distributed, modified, reproduced, republished or
retransmitted in any electronic medium or in hard copy, or derivative work created based on
such images, text, or documents, without express written consent of Beta Bionics.
© 2023 Beta Bionics, Inc. Beta Bionics® and iLet® are registered trademarks of Beta Bionics, Inc. All rights reserved. All other trademarks are
the property of their respective owners. The use of third-party trademarks does not constitute an endorsement or imply a relationship or
other affiliation.
Dexcom, Dexcom Clarity, Dexcom Follow, Dexcom One, Dexcom Share, and any related logos and design marks are either registered
trademarks or trademarks of Dexcom, Inc. in the United States and/or other countries. © 2023 Dexcom, Inc. All rights reserved.
Date of Issuance
2023-05-22
Welcome to the
Beta Bionics family!
The iLet® Bionic Pancreas System is an insulin delivery system that
automatically regulates blood glucose (BG) levels. The iLet System
uses autonomous lifelong learning to calculate and deliver insulin
doses and to continually adapt these doses to your changing insulin
needs.
Read and follow the instructions in this user guide before you start to
use the iLet System.
Need any help? Contact your healthcare provider or contact our Beta
Bionics customer service team.
Important Contacts and Numbers

IV ViLet Bionic Pancreas System User Guide | iLet Bionic Pancreas System User Guide |
Table of Contents
Important User Information 1
About This User Guide 1
Indications for Use 3
Insulin Compatibility 4
Working With Your Healthcare Provider 5
Important Pediatric and Caregiver User
Information 5
General Warnings and Precautions 6
Potential Risks 11
Compatible iCGMs 14
Getting to Know Your
iLet System 15
iLet System Overview 15
Parts of Your iLet System 16
iLet Device 18
Features and Icons 21
Settings Menu 26
History 30
Volume 32
Getting Started With Your
iLet System 33
Preparing to Set Up Your iLet System 34
Setting Up Your iLet System 35
Insulin Set 47
Enter Weight and Go Bionic 49
Living with Your iLet System 50
What to Expect from Your iLet 50
Maintaining your iLet System 51
When Your CGM Sensor is Offline 56
Mobile Device 60
Managing Highs and Lows 62
Meal Announcements 66
Exercise 73
Illnesses 75
Responding to Alerts 76
iLet System Alerts Overview 76
CGM and Glucose Alerts 78
Insulin Delivery Alerts 81
Battery Alerts 81
Reminders 82
Troubleshooting 84
Always Have an Emergency Kit 84
Verify Proper Functionality 85
Care Information 86
General Handling 86
Cleaning Your iLet Device 86
Clinical Performance 87
Introduction 87
Clinical Study Overview 87
Demographics 89
Primary Endpoints 93
Secondary Endpoints 96
Adverse Effects 100
Intervention Compliance 103
Safety of the iLet System using
BG entries only 104
References 104
Technical Information 105
iLet Dosing Decision Software 105
iLet System Specifications (iLet Device,
CGM Sensor, and CGM Transmitter) 108
iLet Device Specifications 109
iLet System Delivery Accuracy 112
Explanation of Symbols 116
Electromagnetic Compatibility 118
Electromagnetic Emissions 119
Electromagnetic Immunity 119
Quality of Wireless Service and
Data Security 123
FCC Notice Concerning Interference 124
Warranty 126
iLet Device Warranty 126
iLet Cartridge Warranty 127
iLet Infusion Set Warranty 128
iLet Connect Warranty 129
Returned Goods Warranty 131

VI 1iLet Bionic Pancreas System User Guide | iLet Bionic Pancreas System User Guide | Important User Information
About This User Guide
1. Important User Information
1.1 About This User Guide
1.1.1 Overview
The iLet Bionic Pancreas System consists of the iLet bionic pancreas (iLet ACE Pump with iLet
Dosing Decision Software), its disposables, a continuous glucose monitor and an infusion set
(see Section 2.2 Parts of Your iLet System for details).
This user guide provides important information on how to operate your iLet Bionic Pancreas
System (iLet System). It provides step-by-step instructions on how to safely set up, manage,
and care for your iLet System. It also provides important safety information including warnings,
and precautions. It also provides the terms of your product warranty.
Read and follow the instructions in this user guide before using your iLet System and
consistently throughout your future use. Changes in equipment, software, or procedures occur
periodically. Information describing these changes will be included in future editions of this
user guide. Contact Beta Bionics to obtain a replacement copy.
WARNING: Do not use your iLet System and its components before reading this user
guide and participating in training. Failure to follow the instructions in the user guide can
result in over/under delivery of insulin. This can cause very low or very high BG, which
could result in serious injury or death.
WARNING: Consult the manufacturer's instructions that accompany your drug product,
insulin infusion set, iCGM, and SMBG for important information on dosage, administration,
proper handling, contraindications, warnings, and precautions.
CAUTION: Touchscreen images and illustrations of the iLet System components in this user guide
are examples only. The specific settings and information presented should not be considered as
suggestions for your individual needs.
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
Page intentionally left blank

23iLet Bionic Pancreas System User Guide | Important User Information iLet Bionic Pancreas System User Guide | Important User Information
About This User Guide Indications for Use
1.1.2 Safety Statements
In this user guide there are two kinds of safety statements:
WARNING: Statement that alerts the user to the possibility of injury, death, or other serious adverse
reactions associated with the use or misuse of the device.
CAUTION: Statement that alerts the user to the possibility of a problem with the device associated
with its use or misuse (i.e., device malfunction).
1.1.3 Abbreviations
Abbreviation Explanation Abbreviation Explanation
BG Blood Glucose GUI Graphical User
Interface
BP Bionic Pancreas MRI Magnetic Resonance
Imaging
CGM Continuous Glucose
Monitor PET Positron Emission
Tomography
iCGM
Integrated Continuous
Glucose Monitoring
System
RF Radiofrequency
CT Computed
Tomography SMBG Self-monitoring blood
glucose
FCC
Federal
Communications
Commission
SN Serial Number
HCP Healthcare Provider CF Correction Factor
iAGC
Interoperable
Automated Glycemic
Controller
ACE Alternate Controller
Enabled
BF Body Floating EMC Electromagnetic
Compatibility
1.2 Indications for Use
The person with diabetes is an intended operator of the iLet bionic pancreas, which consists of
the iLet ACE Pump and the iLet Dosing Decision Software. The iLet bionic pancreas is for use
according to the following:
• For a single person only
• For home use
• For people with type 1 diabetes mellitus
• For people 6 years of age or older
• For use with a compatible iCGM
• For use with a prescription
The Indications for Use for the iLet ACE Pump and iLet Dosing Decision Software are explained
here:
1.2.1 Indications for Use: iLet ACE Pump
The iLet ACE Pump is an alternate controller enabled (ACE) pump intended to deliver insulin
under the skin based on input from an integrated continuous glucose monitor (iCGM) and
an interoperable automated glycemic controller (iAGC), in people 6 years of age or older with
diabetes mellitus. The iLet ACE Pump is intended for single-person use; it is not to be shared.

45iLet Bionic Pancreas System User Guide | Important User Information iLet Bionic Pancreas System User Guide | Important User Information
Insulin Compatibility Working With Your Healthcare Provider
1.2.2 Indications for Use: iLet Dosing Decision Software
The iLet Dosing Decision Software is intended for use with compatible integrated continuous
glucose monitors (iCGM) and alternate controller enabled (ACE) pumps. A self-monitoring of
blood glucose (SMBG) meter may also be used for manual input of blood glucose values to
continue insulin dosing for a limited period of time when input from the iCGM is temporarily not
available.
The iLet Dosing Decision Software autonomously determines and commands an increase,
decrease, maintenance, or suspension of all basal doses of insulin and autonomously
determines and commands correction doses of insulin based on input from an iCGM,
and it autonomously determines and commands meal doses of insulin based on meal
announcements.
iLet Dosing Decision Software is intended for the management of type 1 diabetes mellitus in
people 6 years of age or older. iLet Dosing Decision Software is intended for single patient use
and requires a prescription.
1.3 Insulin Compatibility
The iLet ACE Pump and iLet Dosing Decision Software are designed to use rapid-acting U-100
insulin. The following U-100 rapid acting insulin analogs have been tested and found to be safe
for use in the iLet device:
• NovoLog (insulin aspart) and Humalog (insulin lispro) for ages 6 years and older
NovoLog and Humalog are compatible with the system for use up to 72 hours (3 days). If you
have questions about using other insulins, contact your healthcare provider. Always consult
your healthcare provider and refer to the insulin labeling prior to use.
Please refer to the drug manufacturer’s labeling for drug related information including dosage
and administration contraindications, warnings and precautions.
1.4 Working With Your Healthcare Provider
Your healthcare provider (HCP) can help you establish diabetes management guidelines that
best fit your lifestyle and health needs.
WARNING: DO NOT start to use your system without adequate training from your HCP and/or a
certified iLet trainer. DO NOT change your settings without guidance from your HCP.
WARNING: Monitor your BG with the guidance of your healthcare provider. Improper or inadequate
monitoring may result in undetected hyperglycemia or hypoglycemia.
1.5 Important Pediatric and Caregiver User
Information
The following recommendations are meant to help younger users and others who require a
caregiver and their caregivers to program, manage, and maintain the iLet System.
• It is the responsibility of the healthcare provider and caregiver to decide if the user is
appropriate for treatment with the iLet System.
• Users may accidentally press or tap the touchscreen, leading to unintentional insulin
delivery. Consider using the Limited Access feature, which is an optional, user-settable
passcode, to additionally guard against accidental presses and taps, and to prevent
unauthorized access to the iLet device. For more information about Limited Access, see
Section 2.5.4.3 Limited Access.
• Review the Meal Announcement feature to determine how it best fits with the user's care
plan.
• The insulin infusion set may become dislodged more often with younger users and may
need to be secured. Consult with your child’s healthcare provider about how to safely
secure the components of the iLet System.
WARNING: Keep all parts of the iLet System out of the reach of children. The iLet System contains
small parts (i.e., USB cables, insulin infusion sets with flexible tubing, needles, syringes, and
cartridges). These parts can pose a strangulation or choking hazard or cause internal injury if
swallowed.
67iLet Bionic Pancreas System User Guide | Important User Information iLet Bionic Pancreas System User Guide | Important User Information
General Warnings and Precautions General Warnings and Precautions
WARNING: Do not allow young children to hold the CGM sensor, transmitter, or transmitter kit box
without adult supervision. The sensor and transmitter include small parts that may pose choking
hazards. Keep the transmitter kit box away from young children. The transmitter kit box contains a
magnet that should not be swallowed.
CAUTION: Check the iLet System’s personal settings regularly to make sure they are correct,
especially if the iLet device has been left unattended. Incorrect settings can result in over delivery or
under delivery of insulin.
1.6 General Warnings and Precautions
WARNING: Do not use the iLet ACE Pump and Dosing Decision Software if you are unable or
unwilling to test blood glucose (BG) levels with an SMBG meter when input from the iCGM is
not available.
WARNING: Do not use the iLet ACE Pump and Dosing Decision Software if you are unable or
unwilling to recognize and respond to iLet safety alerts.
WARNING: Do not use the iLet System if you are taking hydroxyurea, also known as Hydrea. This
medication is sometimes used in the treatment of blood disorders and some kinds of cancer. The
use of hydroxyurea can result in falsely elevated sensor glucose readings. The iLet System relies on
sensor glucose readings to adjust insulin, provide insulin doses, and provide high and low glucose
alerts. If the iLet System receives sensor readings that are higher than actual glucose levels, it could
result in missed hypoglycemia alerts and potential errors in diabetes management, such as too
much insulin being delivered. Hydroxyurea can also result in errors when reviewing, analyzing, and
interpreting historical patterns for assessing glucose control.
WARNING: Do not use the iLet ACE Pump and Dosing Decision Software in people under 6
years of age. The iLet ACE Pump and Dosing Decision Software have not been studied in these
populations.
WARNING: Do not use the iLet ACE Pump and Dosing Decision Software in people who are
pregnant, on dialysis or critically ill. The iLet ACE Pump and Dosing Decision Software have not
been studied in these populations.
WARNING: The iLet System is only for use with insulin U-100 lispro (Humalog) and insulin U-100
aspart (Novolog).
WARNING: The iLet System is only for use with the Dexcom G6 iCGM. When using the iLet device,
wear an iCGM.
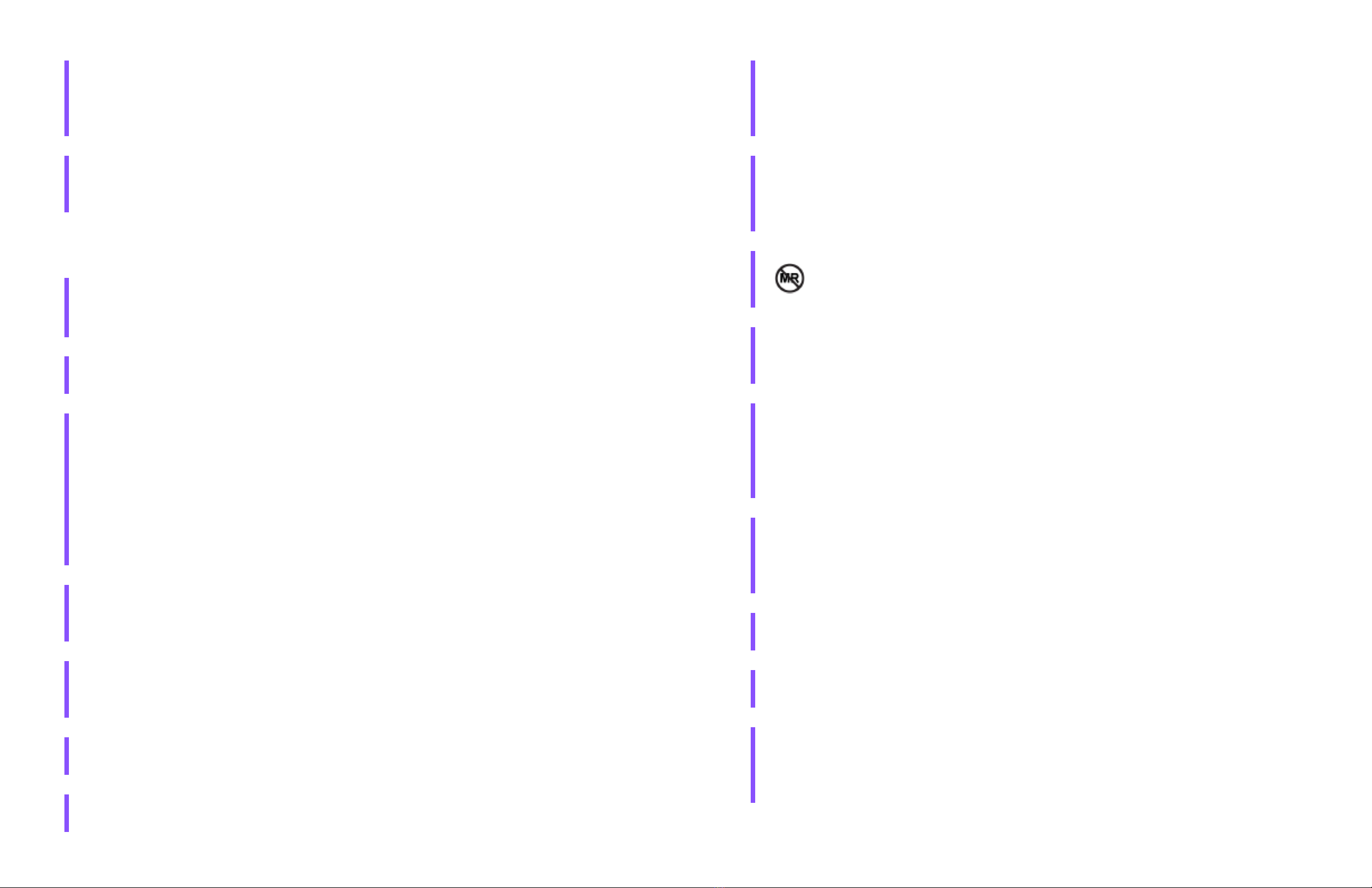
WARNING: Do not expose your iLet System, including your iLet device, steel infusion set, CGM
transmitter, and CGM sensor, to X-ray (screening at airports or other facilities and procedures),
Computed Tomography (CT) scan, Magnetic Resonance Imaging (MRI), or Positron Emission
Tomography (PET) scan.
WARNING: Remove the iLet device, steel infusion set, CGM sensor, and CGM transmitter before
undergoing radiation therapy, Magnetic Resonance Imaging (MRI), Computed Tomography (CT)
scan, or diathermy treatment procedures. Exposure of the iLet device, steel infusion set, CGM
sensor, or CGM transmitter to any of these may damage them.
WARNING: Your iLet System, including your iLet device, steel infusion set, CGM
transmitter, and CGM sensor, is not magnetic resonance (MR) safe. Your iLet System must
be left outside of the procedure room if you are receiving an MRI scan.
WARNING: Do not expose your iLet device, steel infusion set, CGM transmitter, or CGM sensor
to equipment used in procedures for Pacemaker/Automatic Implantable Cardioverter Defibrillator
(AICD) placement or reprogramming, Cardiac Catheterization, or Nuclear Stress Test.
WARNING: Depending on the equipment being used during general anesthesia, your iLet
System may need to be removed. You do not need to remove iLet System components for
electrocardiograms (EKGs) or colonoscopies. Metal detectors and body scanners at airports are
also acceptable. Remove your iLet System prior to any laser surgery as some lasers can create
interference and cause your iLet System to alert you.
WARNING: Do not try to open or repair your iLet device. It is a sealed device that should not be
opened. Modification could result in improper functioning and safety risks. If your iLet device seal is
broken, your iLet device is no longer watertight and the warranty is voided. If you are unsure about
potential damage, discontinue the use of your iLet device and contact Beta Bionics.
WARNING: Your iLet System is for single patient use only. Sharing any part of your iLet System may
lead to transfer of germs, infection, or over/under delivery of insulin.
WARNING: Do not ignore symptoms of hyperglycemia and hypoglycemia. If your sensor glucose
alerts or readings do not match your symptoms, measure your BG with a BG meter.
WARNING: Do not expect CGM alerts when the CGM sensor is warming up up for less than 2
hours. You will NOT get any sensor glucose readings or alerts until the 2-hour warmup ends. During
this time, you might miss severe hyperglycemia or hypoglycemia events. Check your BG with a
meter.

89iLet Bionic Pancreas System User Guide | Important User Information iLet Bionic Pancreas System User Guide | Important User Information
General Warnings and Precautions General Warnings and Precautions
WARNING: The system should NOT be used in hospitalized people as the safety of the technology
has not been evaluated in this population.
WARNING: Use only the AC power adapter and USB cable provided with the iLet device when
charging the iLet device. Use of another power supply could damage the iLet device or create the
risk of fire or burns.
WARNING: Take care when plugging and unplugging your USB cable. Do not force or bend the
end of the USB cable into the charging port.
WARNING: Choose a location for charging where you can easily access the power adapter and
quickly disconnect to prevent the potential risk of electrical shock.
WARNING: Do not expose the USB cable or power adapter to water or other liquids as this may
cause. them to not function properly and may lead to risk of fire or burns.
WARNING: If your AC power adapter or USB cable is damaged or lost, please contact Beta Bionics
Customer Support for a replacement to ensure safe operation of the iLet device.
CAUTION: Avoid exposure of your iLet device to temperatures below 40°F (5°C) or above 104°F
(40°C). Insulin can freeze at low temperatures and degrade at high temperatures. Insulin exposed
to conditions outside of the manufacturer’s recommended ranges can affect the safety and
performance of your iLet System.
CAUTION: Do not place any part of your iLet System in water. If your iLet System has been exposed
to water, check for any signs of water entering your iLet System. If there are signs of water entry, stop
using your iLet System and use an alternative therapy.
CAUTION: Disconnect the tubing set from your body while on amusement park thrill rides. Rapid
changes in altitude or gravity can affect insulin delivery and cause injury.
CAUTION: Disconnect the tubing set from your body before entering an aircraft without cabin
pressurization or in planes used for aerobatics or combat simulation. Rapid changes in altitude or
gravity can affect insulin delivery and cause injury.
CAUTION: Do not separate the CGM transmitter and iLet device by more than 20 feet. The range
from the transmitter to the iLet device is less than 20 feet without obstruction.
WARNING: Do not use your CGM transmitter if it is damaged/cracked. This could cause electrical
safety hazards or malfunction, e.g., electrical shocks.
WARNING: Do not ignore broken CGM sensors or detached sensor wires. If a sensor wire breaks
off under your skin and you cannot see it, do not try to remove it. Contact your healthcare provider.
Seek professional medical help if you have symptoms of infection or inflammation – redness,
swelling, or pain – at the insertion site.
WARNING: Do not insert the CGM sensor in sites other than the belly or upper buttocks (for ages
6–17 only). Other sites have not been studied or approved. Use in other sites might cause inaccurate
sensor glucose readings. This could result in missing severe hyperglycemia or hypoglycemia
events. See the CGM manufacturer’s Instructions for Use for details.
WARNING: Do not inject insulin or insert an insulin infusion set within 3 inches from the CGM
Sensor. The insulin delivered through the insulin infusion set might affect sensor accuracy, resulting
in over/under delivery of insulin. This can cause missing severe hypoglycemia or hyperglycemia
events.
WARNING: Always notify your healthcare provider about your diabetes and your iLet System. If
you need to discontinue the use of your iLet System for medical procedures, follow your healthcare
provider’s instructions on how to disconnect your iLet System.
WARNING: Do not use cartridges other than those manufactured by Beta Bionics or the prefilled
pharmacy dispensed drugs on the recommended list. Use of cartridges not recommended may
affect the performance of your iLet System. It can be unsafe to use accessories, detachable parts
and materials not described in the instructions for use. Secure the iLet device to your body in any
orientation of your choosing to avoid the iLet device falling, dropping or damaging the tubing
line.
WARNING: After installation, do not remove and reinstall the cartridge, tubing line, and/or Luer
connector. If these components are removed from the iLet device, they should be discarded.
Replace the cartridge, tubing line, and/or Luer connector with new components following the
appropriate procedures in this User Guide.
WARNING: Use of accessories, cables, adapters, and chargers other than those specified or
provided by the manufacturer of this equipment could result in increased electromagnetic emissions
or decreased electromagnetic immunity of this equipment and result in improper operation.

10 11iLet Bionic Pancreas System User Guide | Important User Information iLet Bionic Pancreas System User Guide | Important User Information
General Warnings and Precautions Potential Risks
CAUTION: If you disconnect from your iLet, you may need to consider, with guidance from your
healthcare provider, the potential need for carbohydrates relative to the amount of insulin on board
and activity you may engage in. You may view your Insulin On Board within Algorithm Steps under
the History feature. check your BG before disconnecting from and after reconnecting to your iLet
system.
CAUTION: The iLet Mobile App is compatible with the iOS platform and Android platform. The iLet
Mobile App provides the ability to perform over-the-air updates and / or pull data from an iLet device
to share with the Beta Bionics Cloud.
CAUTION: Do not install apps on your smartphone from untrusted sources. These apps may
contain malware that may impact use of the iLet Mobile App. Install apps only from trusted sources
(i.e. Apple App store or Google Play store). If you do not know what an App is, do not install it,
regardless of the source. It is not advised to install any app from a source other than the Apple App
Store or Google Play store on your smartphone that is running the iLet Mobile App. Doing so may
put you at risk of unintentionally installing malware on your device.
CAUTION: Malware, or "malicious software" from unknown third-parties, is designed to damage
your device and/or read your private information. Unknown Apps and unknown downloads are the
most common method for spreading malware. Malware could prevent the iLet Mobile App from
functioning as intended.
CAUTION: Depending on the length of time and reason you disconnect from your iLet System, you
may need to replace missed insulin doses. Treat high and low BG levels as recommended by your
healthcare provider when disconnected from your iLet System.
CAUTION: The iLet Mobile App performs a check to ensure that your device is not rooted,
jailbroken or installed via sideloading. Rooted or jailbroken means the removal of limitations and
security measures set by the manufacturer of a smart device. The removal of these poses a security
risk and data may become vulnerable. Sideloading means the loading of an application from an app
binary file or downloading a file that can install an executable on a smartphone.
If the iLet Mobile App determines your device is rooted, jailbroken and/or has applications installed
via sideloading, you will be blocked from iLet Mobile App use.
CAUTION: If you believe you may have an App installed from a third-party source, take steps to
delete that App. If you believe you may have malware on your device, discontinue use of your iLet
Mobile App, and contact Beta Bionics customer service.
CAUTION: The iLet device cannot connect wirelessly with a self-monitoring blood glucose device,
and manual BG value entries must be performed when the iLet device alerts you for a BG entry.
CAUTION: Bluetooth Low Energy technology is a type of wireless communication used in cell
phones and many other devices. Your iLet device and CGM transmitter wirelessly pair together with
other devices using Bluetooth wireless communication technology. When paired, this allows the
iLet device and CGM transmitter to communicate securely and only with each other.
CAUTION: If your CGM is offline for an extended period of time, dosing will stop and you should
switch to alternative therapy until you are able to reconnect to a CGM sensor. A countdown timer will
appear before dosing would stop.
1.7 Potential Risks
1.7.1 Potential Risks Related to Using Your iLet System
Potential interruption of insulin delivery caused by a system failure (hardware or software
defects) may present risks. These general risks may include:
• Hypoglycemia (low BG)
• Hyperglycemia (high BG)
• Diabetic Ketoacidosis (a potentially life-threatening complication during which the body
produces excess amount of blood acids, called ketones)
• Seizure
• Coma
• Death
Users may accidentally press or tap the touchscreen, leading to unintentional insulin delivery. Consider
using the Limited Access feature, which is an optional, user-settable passcode, to additionally guard
against accidental presses and taps, and to prevent unauthorized access to the iLet device. For more
information about Limited Access, see Section 2.5.4.3 Limited Access.
1.7.2 Potential Risks Related to Using an Insulin Infusion Set
Read and follow the instructions that accompany your insulin infusion set to determine safe

12 13iLet Bionic Pancreas System User Guide | Important User Information iLet Bionic Pancreas System User Guide | Important User Information
Potential Risks Compatible iCGMs
include:
• Local infection
• Bruising
• Bleeding
• Skin irritation, redness, itching, or swelling
• Discomfort or pain
• Rash or skin discoloration
There is a small chance that the CGM sensor wire could break while you are wearing it and
remain under your skin. If you think this occurs, contact your healthcare provider immediately.
You will not get sensor alerts on the iLet device under the following conditions:
• When an alert is snoozed after acknowledgement
• When your transmitter is not within range
• When your iLet device is not receiving sensor glucose readings
• When you are unable to notice the alert or vibration
The CGM takes readings from the fluid below the skin (interstitial fluid), instead of blood.
Measuring glucose in the interstitial fluid (the fluid that surrounds the cells of your tissue below
your skin) differs from measuring it in the blood. Glucose is absorbed into the interstitial fluid
more slowly than it is absorbed into the blood. Therefore, CGM readings lag from the BG meter
readings. Talk to your healthcare provider about the difference between CGM readings and BG
meter readings or refer to the CGM manufacturer's instructions.
1.8 Compatible iCGMs
Compatible CGMs with the ACE Pump and iAGC include the following iCGMs:
• Dexcom G6 CGM
For information about Dexcom G6 CGM product specifications and performance
characteristics, visit the manufacturer’s website.
The Dexcom G6 sensors and transmitters are sold and shipped separately by Dexcom.
and proper handling. General risks related to the insulin infusion set may include:
• Local infection
• Skin irritation, redness, itching, or swelling
• Bruising
• Discomfort or pain
• Bleeding
• Rash or skin discoloration
• Occlusions (blockages) or air bubbles that can interrupt insulin delivery and lead to
hyperglycemia or diabetic ketoacidosis
There is a small chance that an insulin infusion set cannula (the tube that remains after the
insulin infusion set needle is removed) could break and remain under your skin. If that occurs,
contact your healthcare provider immediately.
If an infusion site becomes irritated or inflamed, the insulin infusion set should be removed and
replaced in a new location on your body.
1.7.3 Potential Risks Related to Using a CGM
Read and follow the instructions that accompany your CGM to determine safe and proper
handling, including contraindications, warnings and precautions.
CGM Inaccuracies
• Your iLet device relies on CGM values to dose appropriately. Inaccurate CGM values could
lead to under or over delivery of insulin (e.g., when your BG values are rapidly rising or
falling).
• CGM inaccuracies are usually related to your sensor only and not to your transmitter or
iLet device. If your CGM values do not match your symptoms, always check your glucose
using a SMBG meter. Consider treatment and/or CGM sensor calibration if necessary.
• Your CGM and iLet device will alert you when a CGM calibration is needed.
• Your CGM and infusion set should be placed at least 3 inches apart on the body.
General risks related to CGM sensor use, due to its insertion into the skin or skin adhesive, may

14 15iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System
Compatible iCGMs iLet System Overview
2. Getting to Know Your iLet
System
2.1 iLet System Overview
The iLet Bionic Pancreas System is a closed-loop system that delivers insulin based on input
from an integrated continuous glucose monitor (iCGM) in order to automatically regulate
blood-glucose (BG) levels (see Figure 1). The iLet System uses autonomous lifelong learning to
calculate and deliver insulin doses and to continually adapt these doses to your changing
insulin needs.
Figure 1
16 17iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System
Parts of Your iLet System Parts of Your iLet System
Figure 2
f
e
b
aCannula
1
2
3
c
dg
Flexible tubing
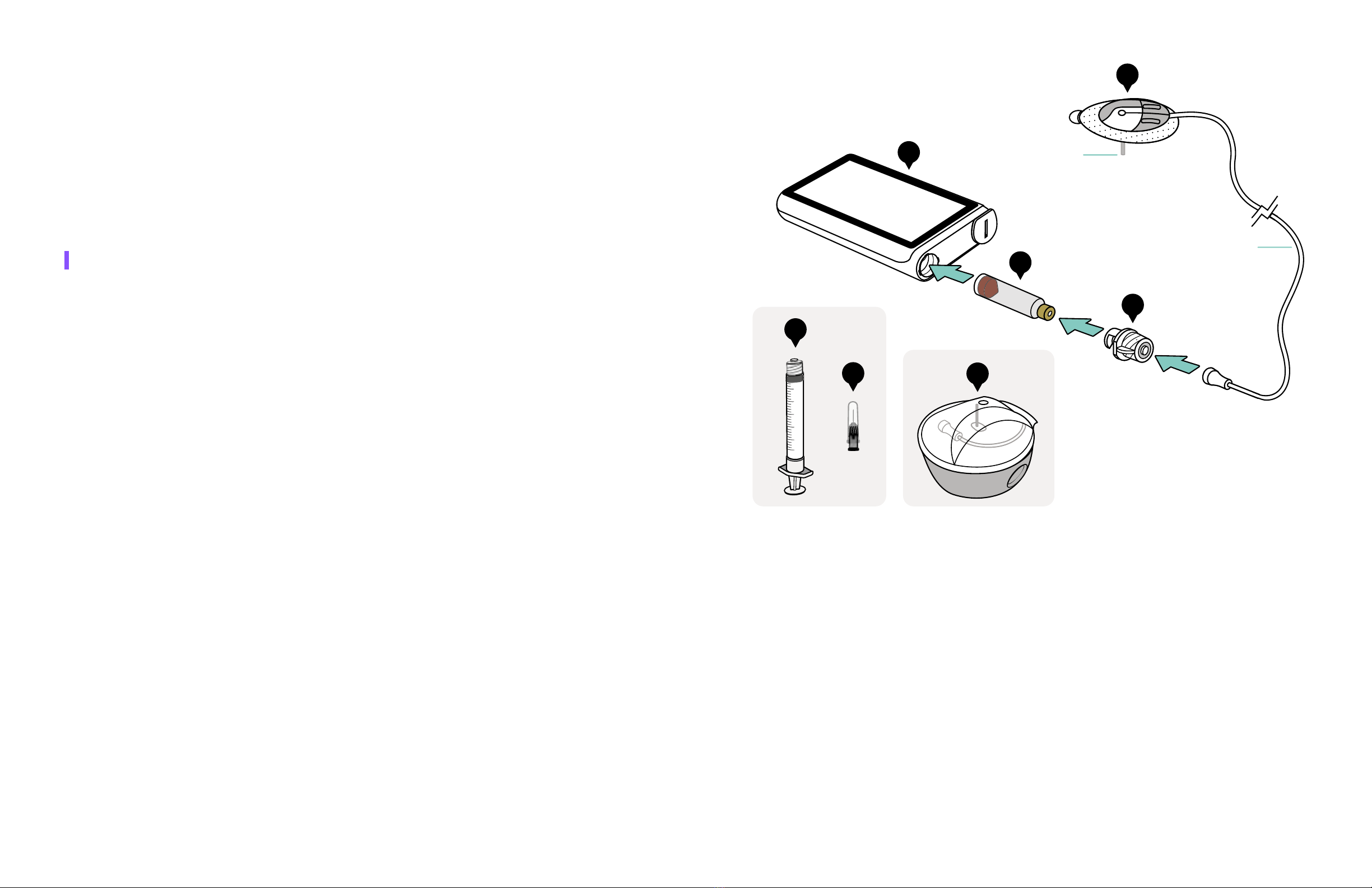
2.2 Parts of Your iLet System
The iLet System consists of the iLet ACE Pump with iLet Dosing Decision Software and
disposable parts (see Figure 2):
a. iLet Device: Device that automatically delivers insulin subcutaneously based on input
from an integrated continuous glucose monitor (iCGM) and/or an SMBG meter, and an
interoperable automated glycemic controller (iAGC). In the absence of input from an iCGM,
the iAGC can instead use blood glucose entries from an SMBG meter.
Replace the following disposable parts (b, e, f, and g) every 2-3 days:
b. iLet Cartridge: Glass container with a soft membrane on top called a septum and a red
rubber plunger. iLet Cartridge is filled with insulin and inserted into your iLet device.
c. Syringe: Plastic syringe (3 mL) that connects to the needle and is used to transfer insulin
from a vial into the cartridge.
d. Needle: Needle (3/8-inch) with needle guard (i.e., protective needle cap).
e. iLet Connect: Plastic Luer connector that attaches the flexible tubing of the insulin infusion
set to your iLet device.
f. Insulin Infusion Set Base: Adhesive patch that sticks on the body with a plastic housing
on top and the tiny tube called a cannula that sits under the skin to deliver insulin. Flexible
tubing connects the insulin infusion set base to the iLet device using the iLet Connect.
g. Insulin Infusion Set: Contains the insulin infusion set base, flexible tubing, and inserter,
and is used to attach the insulin infusion set base to your body
18 19iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System
iLet Device iLet Device
2.3 iLet Device
Figure 3
iLet Charge
Power adaptor
Status light
Micro-USB
cable
Sleep/
Wake Button
Insulin chamber
Touchscreen
It is important to insert your insulin infusion set and CGM in locations on your body per the
recommendations provided by the manufacturer of your insulin infusion set and CGM.
The iLet device may be worn in any orientation. It may be placed in a pocket, or worn
supported in any manner as long as the method of wearing the iLet device does not impede
access for monitoring alerts or cause damage or occlusion to the infusion tubing line.
2.3.1 Charging Your iLet Device
WARNING: Do not run your iLet device on low power for too long. If your iLet device runs out of
power, it will not dose insulin or provide you with CGM values, and the Sleep/Wake button will not
turn the touchscreen on or off. See Section 6.2.1 Troubleshooting Device Power for what to do
if your iLet device has run out of power. If your iLet runs out of power, the time of powering down
and data contents of the alarm system log shall be saved. If the log reaches capacity, your iLet will
alert you, and will also discard the oldest data as newer data is generated. Do not position the power
adapter, USB Cable and charging so that it is difficult to operate the iLet device.
WARNING: Install, remove and handle only dry charging components with dry hands. Make sure
that no liquids are present when charging your iLet device.
WARNING: Use only the AC power adapter and USB cable provided with the iLet device when
charging the iLet device. Use of another power supply could damage the iLet device or create the
risk of fire or burns.
Figure 4 Figure 5
WARNING: Take care when plugging and unplugging your USB cable. Do not force or bend the
end of the USB cable into the charging port.
WARNING: Choose a location for charging where you can easily access the power adapter and
quickly disconnect to prevent the potential risk of electrical shock.
WARNING: Do not expose the USB cable or power adapter to water or other liquids as this may
cause. them to not function properly and may lead to risk of fire or burns.
WARNING: If your AC power adapter or USB cable is damaged or lost, please contact Beta Bionics
Customer Support for a replacement to ensure safe operation of the iLet device.
Your iLet device contains a rechargeable
battery that is not replaceable. Charge the
battery daily to maximize battery lifespan.
It typically takes approximately 2 hours to
charge a depleted battery. A fully charged
iLet device will stay on for 4 to 5 days. Your
iLet device’s battery life depends on the
amount of usage, including the backlight
and the amount of insulin delivered. The
iLet is operational and will dose insulin while
charging. The iLet will play a unique audio
tone when it is placed on a charger and is receiving power.
When charging, maintain an appropriate distance of 7 inches from other magnets and
inductive chargers.
Try to charge at times which will limit the disruption of insulin therapy. For example, charging
while bathing or showering will allow the iLet device to maintain an optimum battery level.
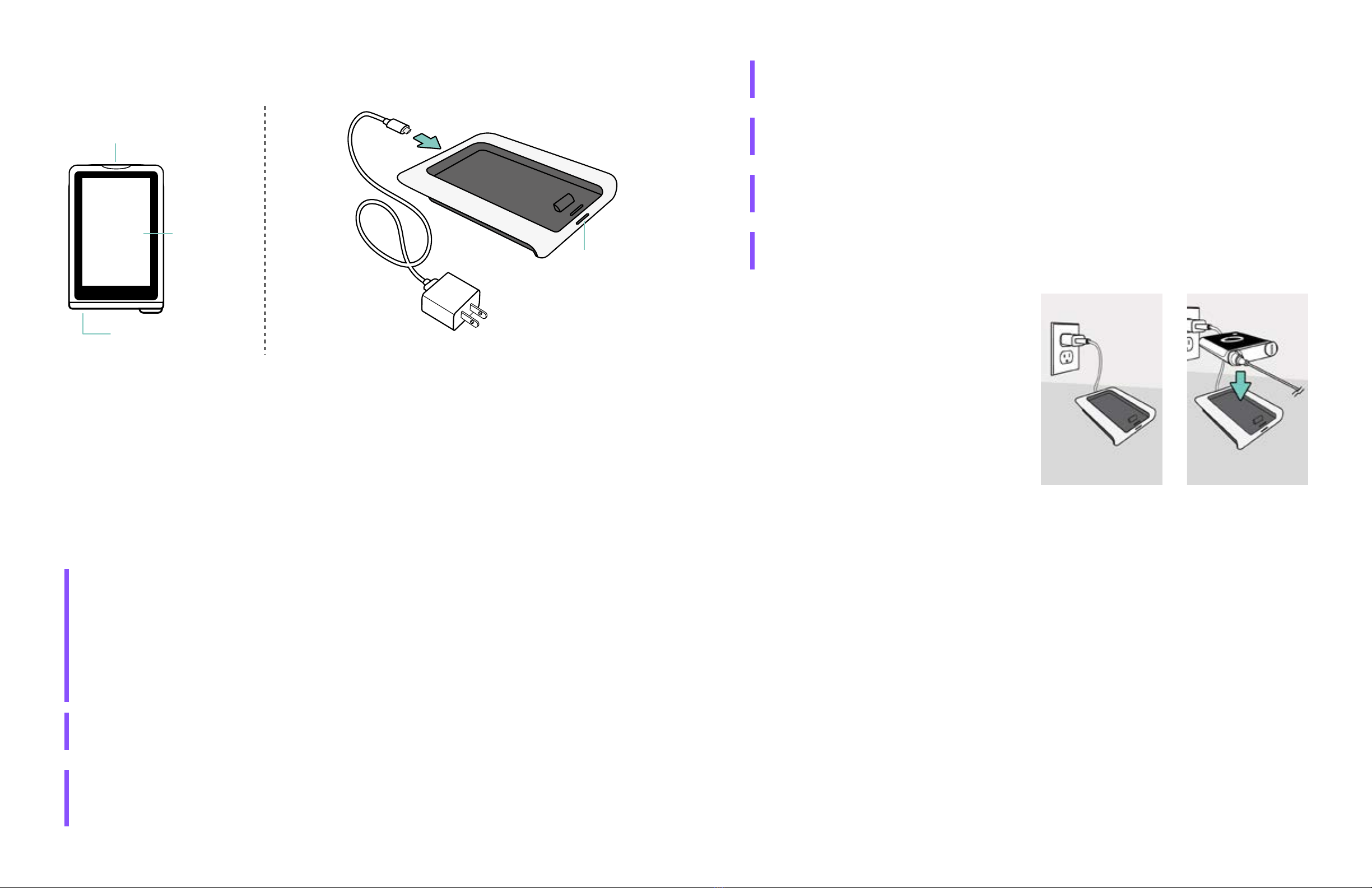
a. Connect the charger to the power adapter with the micro-USB cable and plug it into an
electrical outlet (see Figure 4).
b. Place your iLet device onto the charger (see Figure 5). Be sure to remove your device clip
(iLet Clip) from the iLet device before placing it on the iLet Charge.
c. Make sure that the iLet device is charging properly.
20 21iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System
iLet Device Features and Icons
2.4 Features and Icons
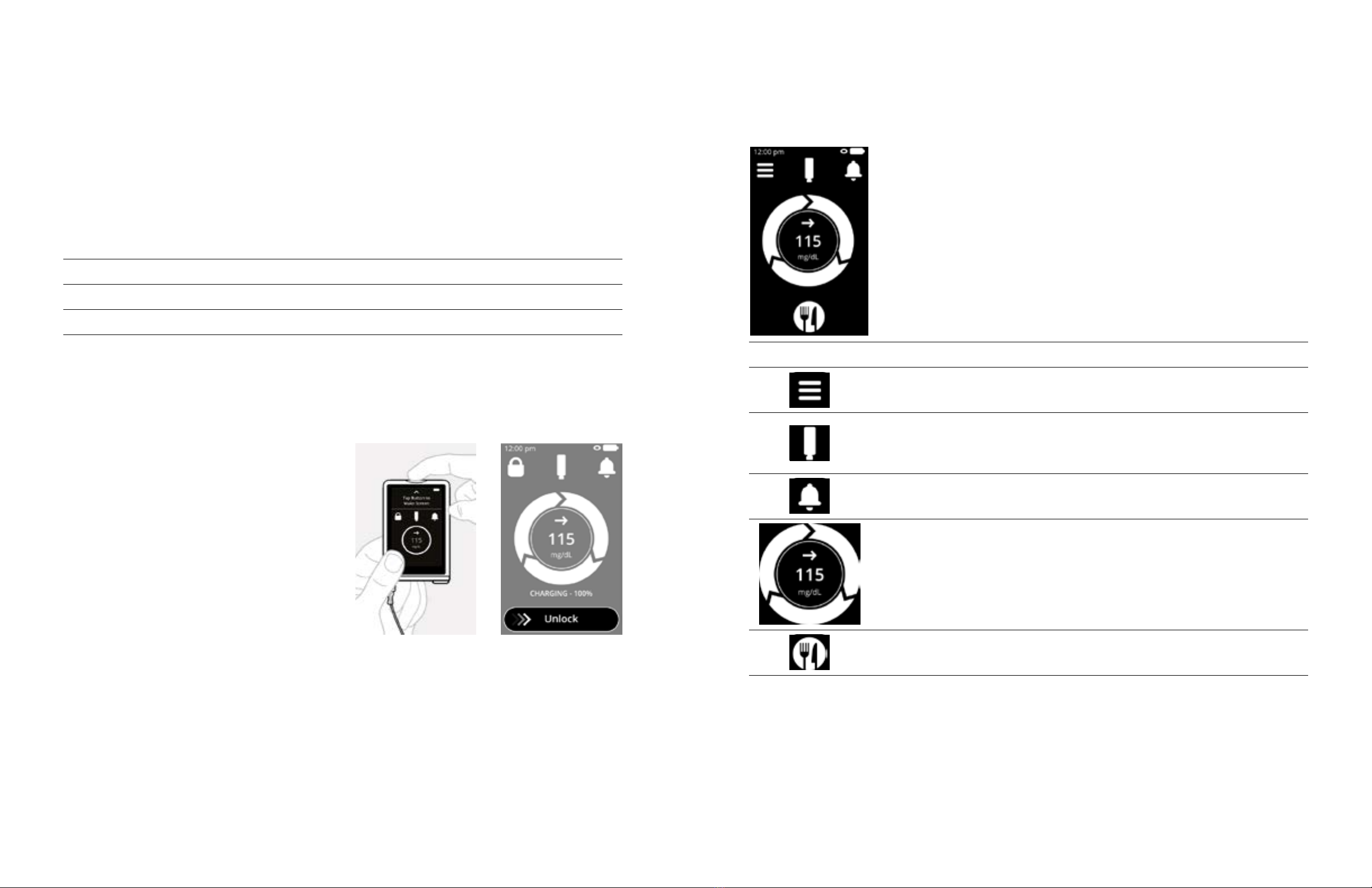
2.4.1 Home Screen
Icon Feature Description
Menu View the Menu screen.
Insulin Cartridge
View the insulin remaining in your cartridge.
Change your insulin cartridge, change your infusion
site, and fill your tubing.
Notifications View and respond to the alerts and reminders for
your iLet System.
Glucose Status
View your current CGM glucose and trend.
The circle will spin when the iLet is running.
Tap to view a graph of CGM and insulin dosing data
and a therapy summary screen.
Meal
Announcement Deliver insulin for meals with carbohydrates.
Figure 8
• If the charger status light is solid and the battery indicator animates on the touchscreen,
your iLet device is charging properly.
• If the charger status light is blinking, your iLet device is not charging properly. Remove your
iLet device from the charger for at least 10 seconds and then place it back on the charger.
2.3.2 Using the Touchscreen
You can navigate the touchscreen with your fingers. Common gestures that you may use to
interact with your iLet device include:
Tap Use your nger to touch an icon on the touchscreen or a button.
Press/Hold Keep pressing a button or icon until its function is complete.
Drag Place your nger on the touchscreen and move it in the direction indicated.
2.3.3 Turning on the Touchscreen and Backlight
The iLet device's touchscreen has a high contrast black and white LCD, with a backlight
available for dark environments.
Always On Touchscreen Display: the
touchscreen will automatically go to sleep
after 45 seconds of inactivity. During this
time, your iLet will continue dosing insulin,
the display will continue to provide basic
status information, but the touchscreen
cannot be activated by finger taps.
To turn the touchscreen on, tap the Sleep/
Wake button (see Figure 6).
To turn the backlight on, lightly press and
hold the Sleep/Wake button for one second.
2.3.4 Unlocking the Touchscreen
Drag the Unlock slider to the right to unlock the touchscreen (see Figure 7).
Figure 6 Figure 7

22 23iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System
Features and Icons Features and Icons
2.4.2 Notifications
Your iLet device will notify you with audio tones and/or vibration alerts
when attention is required. A visible message will appear in the
Notifications icon (see Figure 9). The number of currently active
alerts will appear in the Notifications icon. A bell icon will also
appear in the status bar, next to the battery icon (see Section 5.1 iLet
System Alerts Overview for a list of alerts).
2.4.3 Glucose Trend Arrows
Icon Description
Glucose is steady, and changing less than 1 mg/dL each minute.
Glucose may change up to 15 mg/dL in 15 minutes.
Glucose is slowly rising or falling, and changing 1 - 2 mg/dL each
minute. Glucose may change up to 30 mg/dL in 15 minutes.
Glucose is rising or falling, and changing 2 - 3 mg/dL each minute.
Glucose may change up to 45 mg/dL in 15 minutes.
Glucose is rapidly rising or falling, and changing more than 3 mg/
dL each minute. Glucose may change by more than 45 mg/dL in 15
minutes.
None System can't calculate the speed and direction of your glucose
change.
Figure 9
2.4.4 Glucose Status
Status Description
CGM glucose value will be displayed in the center of the circle. It may
display a trend arrow if enough information is available. The circle will
spin when your iLet System is running.
CGM glucose is below 40 mg/dL. It may display a trend arrow if
enough information is available.
CGM glucose is above 400 mg/dL. It may display a trend arrow if
enough information is available.
CGM sensor has never been paired with your iLet device.
CGM sensor data is not available.
CGM sensor is not connected.
CGM sensor is stopped. CGM glucose value is not available.
CGM sensor is warming up.

24 25iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System
Features and Icons Features and Icons
2.4.5 Graph and Therapy
Summary
Tapping on the CGM glucose value in the
center of the Home screen will display the
Graph screen (see Figure 10). You may tap
this when the device is locked or unlocked.
The Graph screen displays recent CGM
glucose data (small dots) and insulin dosing
(vertical bars).
The CGM chart displays glucose values
between 40 and 400 mg/dL, with an ‘in
range’ section between 70 and 180 mg/dL
(dotted line section in Figure 11).
You can choose between 3, 6, 12, and 24-hour views by tapping on the left and right arrows at
the top of the screen.
Occasionally, small gaps between the CGM glucose values may occur. The gaps represent
periods of missing CGM data. This indicates that the CGM sensor and iLet device have lost
connection briefly. If you notice large gaps between the CGM glucose values, make sure your
iLet device and the CGM sensor are connected. The iLet device will alert you when it has lost
connection with your CGM sensor for 30 minutes or more.
Tap anywhere on the graph to zoom in, and tap anywhere to zoom out.
To toggle to the Therapy Summary screen, tap the chart icon in the top righthand
side of the screen.
The Therapy Summary screen will show summaries of glucose and dosing data (see
Figure 11). You can choose between 1, 7, 30 and 90-day views by tapping on the left
and right arrows at the top of the screen. Tap on the graph icon in the upper
righthand corner to switch back to the Graph screen.
Figure 10 Figure 11
2.4.6 Status Bar
Icon Feature Description
Searching for
CGM or Mobile
Device
Currently searching for a CGM or Mobile Device.
CGM Paired CGM has been paired.
Mobile Device
Paired Mobile device has been paired.
Alert Present An alert is present. View details under the
Notifications feature.
Battery
View the level of your iLet device's battery charge. The
battery icon will display an animation when the iLet is
charging. The home screen will display a % level when
the iLet is charging.
2.4.7 Menu Screen
Tap the Menu icon in the upper left of the
Home Screen to access the menu (see
Figure 12). To close the Menu screen, tap
the Xtab (see Figure 13).
Figure 12 Figure 13

26 27iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System
Settings Menu Settings Menu
2.5.1 About iLet
View details about your iLet device in a scrollable list (see Figure 15). Your iLet device's Serial
Number is located here for reference. The Serial Number may also be found on the back panel
of the iLet device.
NOTE that content on this screen is for demonstrative purposes only and actual content may differ
slightly.
2.5.2 Therapy
Adjust settings which may affect your iLet
device’s dosing (see Figure 16).
2.5.2.1 CGM Target
The default CGM Target setting is Usual.
Adjust the CGM Target to a higher or lower
point (see Figure 17).
CAUTION: Do not adjust the CGM Target or
Sleep CGM Target without your healthcare
provider’s guidance.
CGM Target Numeric Value
Higher 130 mg/dL
Usual 120 mg/dL
Lower 110 mg/dL
Figure 16 Figure 17
Icon Feature Description
Close Close the menu screen.
Enter BG Enter a BG reading and/or calibrate your CGM (see
Section 4.3.2 Enter BG for instructions).
CGM
View options for pairing or managing a CGM
sensor and transmitter with your iLet device (see
Section 4.2.4 Replacing your CGM sensor and
transmitter for instructions).
Mobile
Pair a compatible smart device (phone or tablet)
to your iLet device using the iLet Mobile App (see
Section 4.4 Mobile Device for instructions).
Settings
View and adjust your iLet device settings
(see Section 3.1.2 Enter Your Settings for
instructions).
History
View Alerts, Meal Announcements, Cartridge
Changes, Infusion Set Changes, Algorithm Steps,
and Insulin History (see Section 2.6 History for
instructions).
Volume View and adjust the volume level of your iLet device
(see Section 2.7 Volume for instructions).
2.5 Settings Menu
Settings allow you to adjust
features of your iLet device (see Figure 14).
Figure 14 Figure 15

28 29iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System
Settings Menu Settings Menu
2.5.4 Other
(see Figure 22).
2.5.4.1 Shut Down
Turn off your iLet device for storage. Go into Settings and choose
Other, then choose Shutdown. All dosing will stop. To turn the iLet
device back on, place the iLet device on the charger.
Upon waking up if the battery has been fully depleted, the iLet device
may require you to go through the iLet Startup Sequence again. In
that case, a new treatment session will be started using a new body
weight entry, but all other user settings on the iLet device will be
retained.
2.5.4.2 Restart
Similar to restarting a computer, this will cycle power and restart your
iLet device. Your iLet device’s existing settings, learnings, cartridge
level, CGM session, and autonomous dosing will resume after the
restart is complete.
Figure 22
Figure 23
Figure 24
2.5.2.2 Sleep CGM Target
The default Sleep CGM Target setting is
Usual. Turn on the Sleep CGM Target using
the On/Off toggle (see Figure 18).
This Sleep CGM Target allows you to set a
different target for sleep periods, or other
times of the day. You can customize the start
and end times.
2.5.2.3 CGM Type
Choose the CGM Type that your iLet device will pair with (see Figure 19).
2.5.2.4 Body Weight
Adjust the body weight that the iLet uses to
dose (see Figure 20), if your body weight
changes by more than 15%. Contact your
health provider for guidance.
CAUTION: Do not adjust the body weight
without your healthcare provider’s guidance.
CAUTION: Always check that the body
weight entered is accurate. The iLet will
prompt you to verify the body weight every 3
months.
2.5.3 General
Change device settings (see Figure 21).
Time - Adjust the time displayed on your iLet device’s clock.
Date - Adjust the date of your iLet device.
Language - Choose a language for your iLet device.
Figure 18 Figure 19
Figure 20 Figure 21

30 31iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System
History History
2.5.4.3 Limited Access
Put your iLet device into Limited Access mode. To activate, you will
need to set a passcode between 4 and 8 digits. This function can limit
access to features like meal announcements, cartridges, and settings.
Autonomous dosing will continue while the iLet device is passcode
protected.
If you forget your passcode, please contact Beta Bionics customer
service for more information.
2.5.4.4 Factory Reset
Return your iLet device to factory settings. This will erase all settings,
learnings, and CGM sessions. You will need to set up your iLet device
again (see Figure 26).
2.6 History
Tap the History icon in the Menu to view the event history (see
Figure 27).
2.6.1 Alerts
Tap Alerts to view a scrollable list of alarms over the past 24 hours.
Figure 25
Figure 26
Figure 27
2.6.2 Meals
Tap Meals to view a scrollable list of meal announcements over the past 24 hours.
2.6.3 Cartridges
Tap Cartridges to view information of the last insulin cartridge change. It will also display how
much insulin the tubing was filled with during the last insulin cartridge change.
It will also display how many occlusions (blockages) have occurred since starting the current
algorithm session.
2.6.4 Insulin Infusion Sets
Tap Infusion Sets to view information of the last insulin infusion set change.
2.6.5 Algorithm Steps
Tap Algorithm Steps to view your current Insulin On Board (e.g., how
much active insulin is in your body), a list of the last 36 delivery steps
(3 hours) and the information that was used to calculate the amount of
insulin to deliver. Algorithm Steps provides the following information in
each step:
1. CGM (numeric value)
2. Insulin (dose delivered)
3. Requested Insulin (dose calculated by the algorithm)
4. BG Entered (numeric value, if applicable)
5. Meal Size (carb amount chosen, if applicable)
2.6.6 Insulin History
Tap Insulin History to view a list of iLet insulin history for total daily
basal insulin and meal announcements (see Figure 29).
Figure 28
Figure 29

32 33iLet Bionic Pancreas System User Guide | Getting to Know Your iLet System iLet Bionic Pancreas System User Guide | Getting Started With Your iLet System
Volume Volume
2.7 Volume
You can adjust the volume level of alerts.
More urgent alarms will escalate to the
highest volume level if they are not
acknowledged.
Follow the instructions below to adjust the
volume.
a. From the Home screen, tap the Menu
icon in the upper left corner (see Figure
30)
b. Tap the Volume icon (see Figure 31).
Select the volume you want. Tap any of the four options to play the
volume. iLet will still vibrate when the volume is set to Off, and will still
beep when charging begins (see Figure 32).
Tap Save to confirm selection.
Figure 30 Figure 31
Figure 32
3. Getting Started With Your iLet
System
CAUTION: Check your iLet System’s settings regularly to ensure they are correct. Incorrect settings
can result in over or under delivery of insulin. Consult with your healthcare provider as needed.
CAUTION: Confirm that the correct time and date are set on your iLet device. When editing 12-hour
time, always check that the AM/PM setting is accurate. The incorrect time or date settings may affect
safe insulin delivery.
CAUTION: Confirm that the touchscreen display turns on. You will hear audible beeps and feel your
iLet device vibrate. Confirm that you can see the battery charging indicator on the charger and on
your touchscreen when your iLet device is placed on the charger. If any of these features are not
working, discontinue the use of your iLet System and contact your healthcare provider and Beta
Bionics.
CAUTION: Do not use the vibration feature by itself during sleep unless otherwise directed by your
healthcare provider. Set a high volume for alerts and alarms so you do not miss an important alert or
alarm.
CAUTION: Always look at the touchscreen to confirm you select the correct icon.
CAUTION: Confirm that your CGM transmitter’s serial number (SN) is programmed into your iLet
device before use. Your iLet device cannot communicate with your transmitter unless the correct
CGM transmitter’s SN is entered. If your iLet device and transmitter are not communicating, you will
not receive the sensor’s glucose readings. You might miss alerts regarding severe hypoglycemia or
hyperglycemia events. If you receive a replacement iLet device, make sure that your new device is
programmed with the correct SN.
CAUTION: Do not discard your CGM transmitter when you change your sensor. The transmitter is
reusable. The same transmitter is used with multiple sensors until the transmitter battery life reaches
its end.
CAUTION: Do not use your iLet System if you think your iLet device might be damaged due to
dropping, hitting against a hard surface, or subjecting it to significant vibration. If you are unsure
about potential damage, discontinue the use of your iLet System and contact your healthcare
provider.
Table of contents
Other Beta Bionics Medical Equipment manuals
Popular Medical Equipment manuals by other brands
Datex-Ohmeda
Datex-Ohmeda S/5 Avance Technical reference manual

Tecno-gaz
Tecno-gaz Free 3 Instructions for use

Bestcare
Bestcare BestStand SA600 manual

Gate
Gate Bure Standard EL 56-312T Instructions for use

Stryker
Stryker BERTEC GOBED PLUS FL20E Operation manual

inhealth
inhealth Blom-Singer Instructions for use