Bio-Works BabyBio S User manual

IN 45 100 010 •AA •1 / 12
INSTRUCTION IN 45 100 010
BabyBio S
BabyBio Q
BabyBio DEAE
BabyBio™ S, BabyBio Q and BabyBio DEAE are ready-to-use ion exchange chromatography columns
for easy and convenient purification of proteins, peptides and oligonucleotides, by utilizing the
difference in these molecules surface charge. BabyBio S works as a strong cation exchanger, BabyBio
Q as a strong anion exchanger and BabyBio DEAE as a weak anion exchanger. The columns are
prepacked with WorkBeads™ 40S, WorkBeads 40Q and WorkBeads 40 DEAE resins, and are available
in two column sizes, 1 ml and 5 ml.
Prepacked ready-to-use columns for fast and reliable results
High binding capacity and purity
Easy-to-use for screening of conditions
Short protocol
This general short protocol is for the use of BabyBio S, BabyBio Q and BabyBio DEAE columns. Detailed
instructions and recommendations for optimization are given later in this instruction. Recommended and useful
buffers are listed in Table 3. BabyBio S columns are suitable for basic proteins, i.e. proteins with a high isoelectric
point (pI), while BabyBio Q and BabyBio DEAE columns are suitable for purification of acidic proteins, i.e.
proteins with low pI.
1. Choose a suitable pH and buffer for the binding of the target protein. One pH unit below pI (BabyBio S
columns) or above pI (BabyBio Q and BabyBio DEAE columns) is a good starting point.
2. Connect the column to the chromatography system, syringe or pump.
3. Equilibrate the column with 10 column volumes (CV) 20 - 50 mM binding buffer at the chosen pH.
4. Apply a clarified sample to the column at low ionic strength and the chosen pH (similar to the binding
buffer) to allow binding of the target protein.
5. Wash the column using 10 - 20 CV binding buffer.
6. Elute the target protein.
Alternative 1: Desorb the target protein with 5 CV elution buffer.
Alternative 2: For increased purity, gradient elution is recommended. For example, use a gradient from
0 to 100% with 20 CV binding buffer including 1 M NaCl.
7. Clean the column using 0.5 - 1.0 M NaOH for 15 - 30 minutes (optional).
8. Wash the column with 5 CV deionized water to remove the cleaning solution.
9. Equilibrate with 10 CV 20% ethanol for storage. Close the column using the included cap and plug.
IN 45 100 010 •AA •2 / 12
Principle
Ion exchange chromatography (IEX) can be used for purification of biomolecules, such as proteins, peptides and
oligonucleotides, by utilizing the difference in their surface charge. The biomolecules interact with the
immobilized ion exchange groups with opposite charge on the chromatography resin. WorkBeads resins are
available with sulfonate groups (WorkBeads 40S), quaternary amines (WorkBeads 40Q) or tertiary amines
(WorkBeads 40 DEAE) as the ion exchange groups. WorkBeads 40S is a strong cation exchanger and will bind
positively charged molecules. WorkBeads 40Q and WorkBeads 40 DEAE are strong and weak anion exchangers
respectively and will bind negatively charged molecules. The structure of the ligands used in WorkBeads 40S,
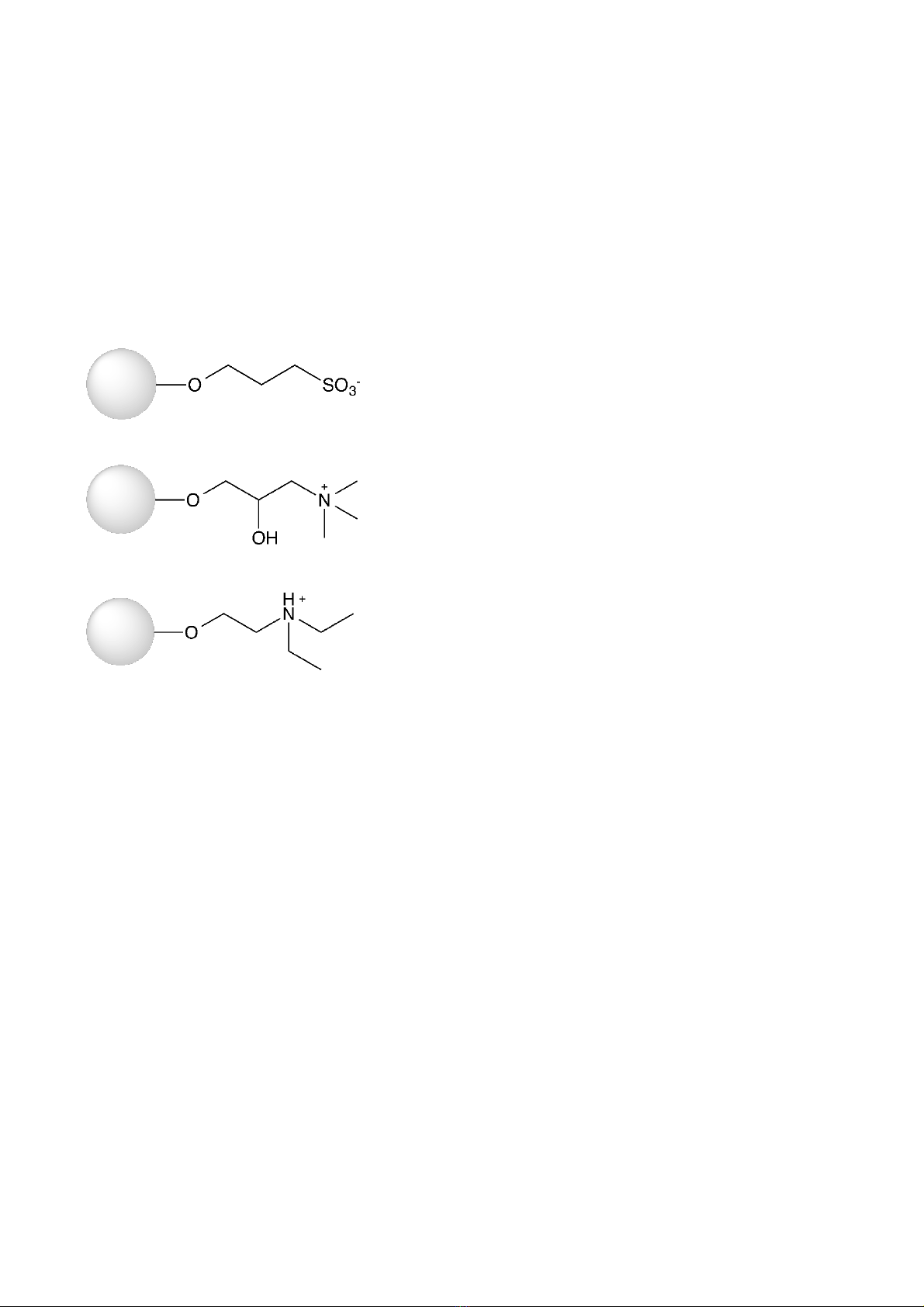
WorkBeads 40Q and WorkBeads 40 DEAE are shown in Figure 1.
(A)
(B)
(C)
Figure 1. Structure of the ligand used in WorkBeads 40S (A), WorkBeads 40Q (B) and WorkBeads 40 DEAE.
The surface charge of proteins depends on the pH of their environment. When the pH is equal to the isoelectric
point (pI) of the protein the net charge is zero. At pH values below the pI the net charge will be positive, and at
a pH above the pI the net charge will be negative. It should be noted that the interaction of the protein depends
on the presence and distribution of both positive and negative charged groups on the surface (net charge). A
protein may therefore interact with an ion exchange resin also at the pI. The likelihood of binding to either the
cation or the anion exchange resin will increase when moving away from the pI.
Ion exchange chromatography begins with equilibration of the column to establish the desired pH and charging
the resin with suitable counter ions to the charged ligands on the resin (e.g., the negative sulfonate groups can
interact with Na+ions and the positive trimethyl amine groups can interact with Cl-ions). It is common to use
an equilibration buffer composed of a buffer substance to control the pH, and NaCl to include suitable counter
ions. When the sample is applied, proteins with suitable charge will bind to the charged groups of the resin
while displacing the counter ions. Desorption of the proteins (elution) is carried out by increasing the
concentration of counter ion (salt gradient elution). The counter ions will displace the proteins as the salt
concentration increases. Various additives (e.g., enzyme inhibitors, non-ionic detergents, urea and low
concentrations of organic solvent) can be used in samples and buffers for IEX as long as they do not strongly
interact strongly with the charged groups on the resin or on the protein which could interfere with the protein
binding to the resin.
Ion exchange chromatography is one of the most frequently used chromatography techniques because of its
versatility and ability to separate proteins even with small differences in charge. It is also one of the more cost-
effective chromatography techniques and is therefore excellent for scale-up.

IN 45 100 010 •AA •3 / 12
Instructions
Purification can be carried out at room temperature or at temperatures down to 4°C. Operation at a low
temperature may require a reduced flow rate due to the increased viscosity of the buffer. All steps can be
carried out with a syringe, a peristaltic pump or a chromatography system. If the chromatography system has
a pressure limit functionality, set the maximum pressure over the column (resin bed) to 3 bar (remember to
take the system fluidics contribution to the pressure into account).
1. Prepare the sample
After cell disruption or extraction, clarify the sample by centrifugation at 10 000 - 20 000 × gfor 15 - 30 minutes.
It is generally recommended to pass the sample through a 0.22 - 0.45 µm filter (e.g. a syringe filter) to avoid
inadvertently applying any remaining particles onto the column. If the sample contains only small amounts of
particles, centrifugation may be omitted and it is enough only to carry out filtration. Application of a sample
that has not been properly clarified may reduce the performance and lifetime of the column. The sample should
be applied under conditions similar to those of the binding buffer.
2. Connect the column
Cut off or twist off the end at the outlet of the column, see Figure 2. Note: It is of high importance to cut off the
tip at the very end of the cone, preferable using a scalpel. Incorrect removal of the end piece will affect the
performance of the column.
Connect the column to your equipment using the recommended connectors shown in Table 1. Fill the
equipment with deionized water or buffer and make drop-to-drop connection with the column to avoid getting
air into the column. Carry out all steps, except for sample application, at 1 ml/min (BabyBio 1 ml column) or 5
ml/min (BabyBio 5 ml column).
Figure 2. Removal of the cut-off end at the column outlet should be done by cutting or by twisting (A) not bending (B).
Table 1. Recommended connectors for coupling BabyBio columns to the equipment of choice.
Equipment
Accessories for connection
Syringe
Female luer or male coned 10-32 threads
Chromatography system
Fingertight connectors (coned 10-32 threads) for 1/16” o.d. tubing

IN 45 100 010 •AA •4 / 12
3. Remove the storage solution
The column contains 20% ethanol on delivery. This storage solution should be washed out before use. Wash
the column with 5 CV deionized water or buffer. Avoid flow rates higher than 2 ml/min for BabyBio 1 ml columns
or 10 ml/min for BabyBio 5 ml columns before the storage solution has been removed to avoid overpressure
due to the relatively high viscosity of the 20% ethanol solution.
4. Equilibrate the column
Equilibrate the column with 10 CV of binding buffer. The buffer should be selected to provide a good buffering
capacity, with pKawithin 0.5 units from the intended pH for capturing the target protein on the selected ion
exchange chromatography column. Examples of buffers to be used are listed in Table 3.
Note: To avoid bacterial growth and poor column performance, use only freshly prepared and filtered buffers.
5. Apply the sample
Apply the sample at 0.5 - 1 ml/min for the BabyBio 1 ml or 2 - 5 ml/min for the BabyBio 5 ml columns. A too
high flow rate may reduce the yield.
Applied samples should have a pH that gives the target protein a charge that is opposite the charge of the
column resin. The pH together with the ionic strength in the sample solution might need adjustment for optimal
binding.
6. Wash
After sample application, remove unbound impurities by washing the column with 20 - 30 CV washing buffer or
until desired A280 nm absorbance of the wash fractions (e.g., 0.01 - 0.02) is obtained.
7. Elute
Alternative 1: Desorb the target protein with 5 CV elution buffer.
Alternative 2: For high purity, gradient elution is recommended. For example, use a linear gradient from 0 to
100% with 20 CV binding buffer including 1 M NaCl.
A stringent wash step with, for instance, 2 M NaCl can be included after elution, to ensure desorption of all
interacting proteins.
8. Re-equilibrate
Clean the column with 0.5 - 1 M NaOH for 15 - 30 minutes and re-equilibrate the column with 10 CV binding
buffer to restore the pH. If additional separation will be carried out, restart at step 5.
9. Column storage
Wash the column with 5 CV deionized water to remove the remaining buffer. Equilibrate the column with 10
CV 20% ethanol for storage. Close the column using the cap and plug (included).
IN 45 100 010 •AA •5 / 12
Scale-up
BabyBio S 1 ml, BabyBio Q 1 ml and BabyBio DEAE 1 ml can be used for purification of up to 50 - 100 mg of
protein. Scale-up from a BabyBio 1 ml column can be carried out by using a BabyBio 5 ml column and applying
a sample volume five times larger. BabyBio columns can be connected in series with a maximum of five columns
(column stacking). This will increase the total capacity of the run accordingly. By connecting BabyBio columns
in series, any column volumes from 1 ml to 25 ml can be obtained. This means a binding capacity of 1000 - 2000
mg of protein can be achieved. Column size selection should be based on estimated amount of target protein
in each run.
Another easy-to-use alternative for scaling up is by using the prepacked OptioBio™ 40S 10x100 or OptioBio 40Q
10x100 columns. OptioBio are prepacked glass columns with WorkBeads 40S or WorkBeads 40Q and with a
column volume of 7.9 ml, see Related products.
BabyBio columns are easily connected together without accessories. Up to five columns may be connected in
series (column stacking). The pressure drop across each column bed will be the same as for a single column,
but the upstream columns will be subjected to a higher internal pressure from the added pressure drops from
downstream columns. It may therefore be necessary to decrease the flow rate accordingly in order to avoid
exceed the maximum pressure limit onto the first column. If possible, the maximum pressure of the
chromatography system should be set according to Table 2. Remember always to take the system fluidics
contribution to the pressure into account.
Table 2. Recommended maximum pressure settings for BabyBio columns connected in series. Notice that the maximum pressure over each column is
always 3 bar.
No. of columns in series
Max pressure BabyBio 1 ml
(bar)
Max pressure BabyBio 5 ml
(bar)
1
3.0
3.0
2
6.0
6.0
3
9.0
9.0
4
12
101
5
15
101
1 The maximum pressure is defined by the column hardware maximum pressure.
Column size selection should be based on the estimated amount of protein to be purified. A test run with a
defined small volume of sample on a BabyBio 1 ml column should be used to estimate the concentration of the
target protein in the sample. A general recommendation is to use 70 - 80% of the column binding capacity. For
large sample volumes with low concentrations of the target protein, it may be suitable to use a larger column
than the one calculated to allow higher sample flow rates, and consequently shorter application time. For
example, using a 5 ml column instead of a 1 ml column allows a flow rate five times higher due to the larger
cross-section of the column. Have in mind that too high flow rate may reduce binding capacity.
For columns larger than 20 ml, it is recommended to pack a single column using bulk resin as the limitations of
column stacking will then impact chromatographic performance. To find out more about Bio-Works bulk
chromatography resins, please visit www.bio-works.com

IN 45 100 010 •AA •6 / 12
Optimization
The following paragraphs will give indications on some parameters that can be tuned to find the optimal
conditions for the purification.
Buffer selection
Choosing a buffer with optimal binding and elution conditions for the target protein will improve the result of
the purification. The buffer should be selected to provide an optimal capacity and with a pKa-value within 0.5
units from the intended pH. Table 3 shows examples of buffers that can be used for ion exchange
chromatography, however the buffer choice will be depending on the target molecule and aim of the
purification procedure. For other useful buffers and their pKa-values at 25 °C see reference: Methods in
Enzymology, Volume 463, pp 46-47, Burgess, R.R and Deutcher M. P.
Table 3. Example of buffers for model protein purification using BabyBio S, BabyBio Q and BabyBio DEAE. Other buffers can possible be used.
Buffer
Product
Buffer composition
Binding buffer
BabyBio S
50 mM Na-phosphate, pH 7.0
Binding buffer
BabyBio Q
50 mM Tris-HCl, pH 7.4
Binding buffer
BabyBio DEAE
50 mM Tris-HCl, pH 7.4
Elution buffer
BabyBio S
50 mM Na-phosphate, 1 M NaCl, pH 7.0
Elution buffer
BabyBio Q
50 mM Tris-HCl, 1 M NaCl, pH 7.4
Elution buffer
BabyBio DEAE
50 mM Tris-HCl, 1 M NaCl, pH 7.4
Preferably, select buffer substances with opposite charge to the resin. A buffer substance that interacts with
the charged groups in the resin may cause local pH disturbances that disturbs the separation. Usually, low
conductivity in the binding buffer is preferred but optimization with regard to pH and conductivity can improve
binding capacity. An increase in ionic strength may decrease the ability of contaminants bind while the target
protein remains bound. However, chromatographic conditions selection should be such that the target protein
is stable during purification.

IN 45 100 010 •AA •7 / 12
Optimization of binding
The key conditions to be optimized is usually pH and conductivity (by addition of NaCl, other salts, or dilution).
Conditions selection should be to maximize purity and/or yield of the target protein, while keeping it in a
native/active state.
pH and salt optimization
Strong ion exchangers (S and Q) are used over a broad pH range. The useful pH range is limited by the target
protein pH stability and solubility window. Weak ion exchangers have a narrower pH range for usage. The weak
DEAE anion exchanger must be used at a pH below its pKavalue of 9.0 - 9.5 to retain its positive charge. The
difference between the Q and DEAE ligands may give desired differences in selectivity. Purification is often done
by combining an anion exchange column and a cation exchange column with or without changing the pH.
It should be noted that the binding capacity and purity depends on the combination of pH and counter ion
concentration (i.e., salt concentration or ionic strength). Therefore, it is recommended to investigate the
combination effects of pH and salt concentration during optimization. A low salt concentration is considered to
give strong binding with high capacity, but it should be noted that it is often observed that an intermediate
concentration of salt gives a better binding capacity. For example, a concentration of 50 mM NaCl in the buffer
may give a better capacity than 20 mM NaCl. This may be attributed to improved mass transport of target
substance into the pores of the resin, obtained by reduced pore exclusion. Pore exclusion can be explained as
a hindrance of diffusion caused by strong interaction of substance on the walls of the outer pores, causing, in
effect, a “traffic jam” and thus reduced diffusion rate into the pores. A slightly elevated salt concentration
reduces, but does not eliminate the interactions with the resin by creating a dynamic adsorption-desorption
equilibrium that allows further diffusion into the resin, thus increasing the binding capacity.
Tuning the flow rate
Flow rate is another factor that can be optimized to improve the binding capacity during sample application or
the resolution during elution. A low flow rate during sample application promotes binding capacity since more
time is allowed for mass transport of the target substance into the pores of the resin. A small substance (e.g., a
peptide) has a high diffusion rate and is not hindered by the walls in the pores and will have fast mass transport
into the resin and thus be adsorbed at a high flow rate. A large target substance (e.g., a large protein) has a
lower diffusion rate and is held back by the walls of the pores slowing its mass transport. A high binding capacity
of this substance may require a lower flow rate. If only a part of the binding capacity of the resin is used the
sample application can be done at a higher flow rate without loss of the target substance.
For scale-up planning it is useful to use the expression residence time instead of flow rate. The residence time
can be defined as the time between entering and exiting the column of a specific part of the sample or buffer.
It can be calculated as column volume divided by the volumetric flow (e.g., the residence time for 1 ml column
at 0.5 ml/min is 1 ml / 0.5 ml/min = 2 minutes). The residence time is typically 1 to 5 minutes in IEX. When a
suitable residence time has been selected using BabyBio Q, BabyBio S or BabyBio DEAE columns, this value can
be used for calculation of a suitable flow rate on a larger column with higher bed. The linear flow rate can be
increased if the bed height is increased while keeping the residence time constant.
IN 45 100 010 •AA •8 / 12
Optimization of washing and elution
Prolonged wash
A continuously decreasing UV signal during washing is an indication of unbound material being washed out (it
may be target substance if it is weakly bound). Washing should be continued until the UV absorbance signal has
reached 0.01-0.02 (“10-20 mAU”). A BabyBio column should be washed with at least 10 CV buffer.
Optimizing elution conditions
Elution can be carried out using a high salt concentration or by altering the pH to change the charge of the
adsorbed protein. A stronger binding may require higher salt concentration for elution.
Step elution
The optimal salt concentration is dependent on purity and recovery requirements as well as properties of the
target protein and the sample. Using a gradient elution gives increased purity than step elution, but step elution
may be necessary to obtain the highest possible concentration of the target protein. In order to optimize the
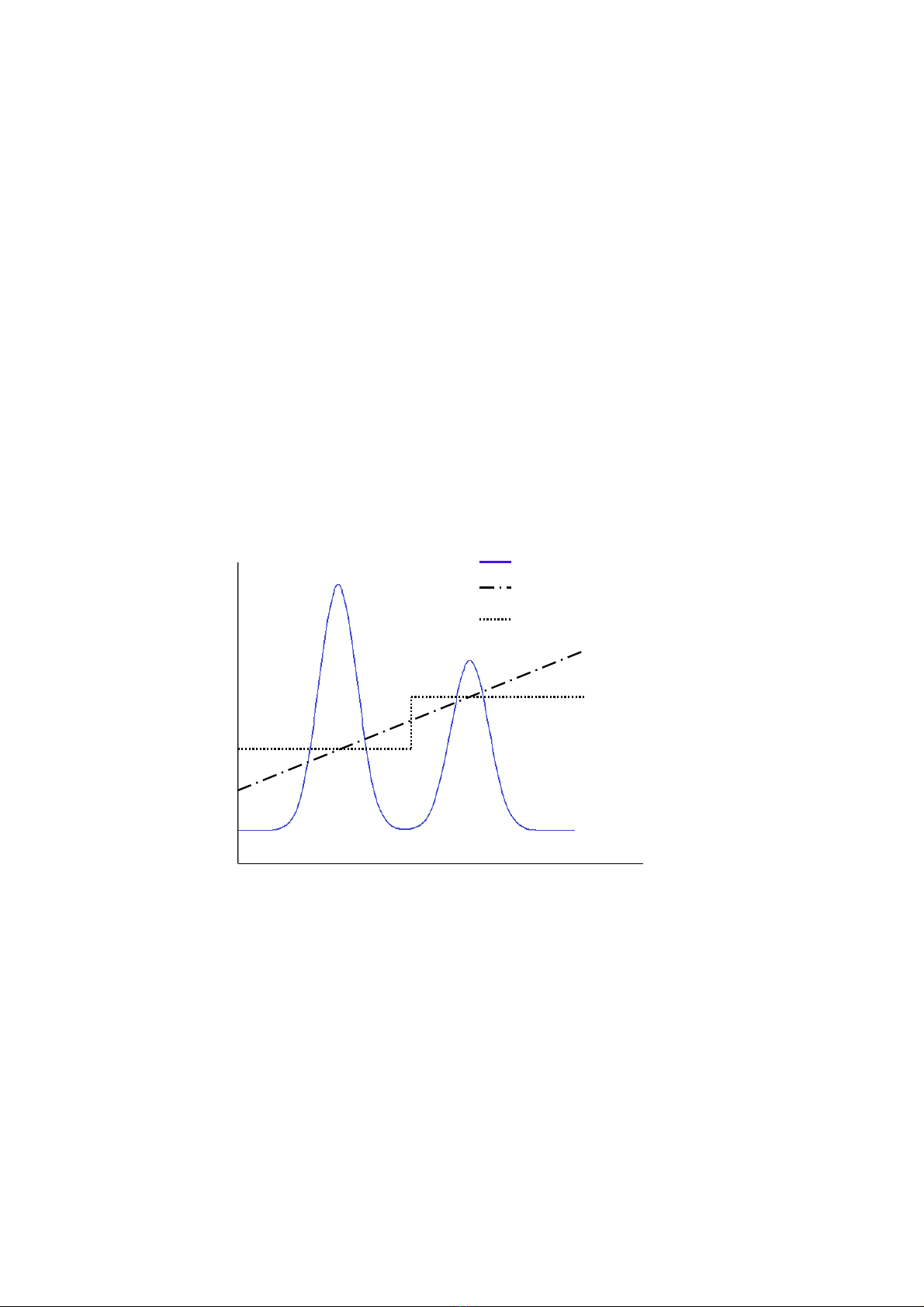
salt concentration for step elution an initial gradient test run can be carried out to identify a suitable step elution
conditions for purification of the target protein, see Figure 3.
Note: Remember to take the system dead volume into account when comparing the print out of the gradient
and the trace.
Figure 3. Optimization of step gradient elution with salt. A test run with linear gradient elution gives information about suitable salt concentrations to
be used during the step elution.
Conductivity, gradient elution
UV absorbance
Conductivity, step elution
Volume

IN 45 100 010 •AA •9 / 12
Extra purification step
Optimization of the overall purification process by tuning the binding, washing and/or elution steps is an option.
However, an additional purification step based on another chromatography technique is recommended (see
Additional purification).
Desalting and buffer exchange
Buffer exchange or desalting of a sample are useful before analysis and/or after chromatography steps such as
ion exchange chromatography. This can be carried out quickly and easily using BabyBio Dsalt 1 ml or 5 ml
columns (see Related products). BabyBio Dsalt columns are also a useful alternative to dialysis for larger sample
volumes or when samples need to be processed rapidly to avoid degradation.
Additional purification
Ion exchange chromatography is a powerful single protein purification step or combined with other
chromatography techniques. The overall process needs to be optimized for each purification step.
To find out more about Bio-Works chromatography resins for additional purification, please visit www.bio-
works.com

IN 45 100 010 •AA •10 / 12
Maintenance of the column
Unpacking and inspection
Unpack the shipment as soon as it arrives and inspect it for damage. Promptly report any damage or
discrepancies to your local supplier.
Cleaning
During purification impurities such as cell debris, lipids, nucleic acids and protein precipitates from the samples
may gradually build up in the resin. The severity of this process depends on the type of sample applied to the
column, and the pre-treatment of the sample. The impurities may reduce the performance of the column over
time. Regular cleaning (Cleaning-in-place, CIP) keeps the resin clean, reduces the rate of further contamination,
and prolongs the capacity, resolution and flow properties of the column. Cleaning of a resin using 1 M NaOH
applied by a low reversed flow for 2 hours or overnight is often sufficient.
CIP of the column can be carried out as followed:
1. Wash the column with 5 CV deionized water.
2. Apply 3 - 10 CV of 0.5 - 1 M NaOH for 15 - 30 minutes.
Note: The contact time is the important factor, treatment with NaOH overnight can be necessary if
severely fouled.
3. Wash the column with 5 - 10 CV deionized water or binding buffer (until the column is neutral after CIP).
4. Equilibrate the column with 10 CV 20% ethanol (for storage).
Sanitization (reduction of microorganisms) can be done using combinations of NaOH and ethanol (e.g.,
incubation with a mixture of 0.5 M NaOH and 40% ethanol for 3 hours). The sanitization procedure and its
effectiveness will depend on the microorganisms to be removed, and needs to be evaluated for each case.
Storage
Equilibrate the column with 20% ethanol and close it securely using the included plug and cap. Store the column
at 2 to 25 °C.

IN 45 100 010 •AA •11 / 12
Additional information
Intended use
BabyBio S, BabyBio Q and BabyBio DEAE columns are intended for research and process development only. The
columns shall not be used for the preparation of material for clinical or diagnostic purposes.
Safety
Please, read the associated Material Safety Data Sheets (MSDS) for BabyBio columns, and the safety instructions
for any equipment to be used. Note that the maximum back pressure of BabyBio S, BabyBio Q and BabyBio
DEAE columns is 0.3 MPa (3 bar, 43 psi).
Product information
BabyBio S
BabyBio Q
BabyBio DEAE
Target substance
Proteins, peptides
Protein, peptides,
oligonucleotides
Protein, peptides,
oligonucleotides
Resin
WorkBeads 40S
WorkBeads 40Q
WorkBeads 40 DEAE
Matrix
Rigid, highly cross-linked
agarose
Rigid, highly cross-linked
agarose
Rigid, highly cross-linked
agarose
Average particle size1(DV50)
45 µm
45 µm
45 µm
Ligand
Sulfonate
(-SO3-)
Quarternary amine
(-N+(CH3)3)
Diethylaminoethyl
(-CH2CH2N+H(CH2CH3)2)
Ion capacity
0.18 - 0.25 mmol Na+/ml resin
0.18 - 0.25 mmol Cl-/ml resin
0.11 - 0.16 mmol Cl-/ml resin
Dynamic binding capacity
130 mg BSA/ml resin2
50 mg BSA/ml resin3
40 mg BSA/ml resin3
Column volume
1 ml
5 ml
1 ml
5 ml
1 ml
5 ml
Column dimension
7 x 28 mm (1 ml)
13 x 38 mm (5 ml)
7 x 28 mm (1 ml)
13 x 38 mm (5 ml)
7 x 28 mm (1 ml)
13 x 38 mm (5 ml)
Recommended flow rate
BabyBio 1 ml
BabyBio 5 ml
1 ml/min (150 cm/h)
5 ml/min (225 cm/h)
1 ml/min (150 cm/h)
5 ml/min (225 cm/h)
1 ml/min (150 cm/h)
5 ml/min (225 cm/h)
Maximum flow rate
BabyBio 1 ml
BabyBio 5 ml
5 ml/min (780 cm/h)
20 ml/min (900 cm/h)
5 ml/min (780 cm/h)
20 ml/min (900 cm/h)
5 ml/min (780 cm/h)
20 ml/min (900 cm/h)
Maximum back pressure
0.3 MPa, 3 bar, 43 psi
0.3 MPa, 3 bar, 43 psi
0.3 MPa, 3 bar, 43 psi
Chemical stability
Compatible with all standard aqueous buffers used for protein purification and 70% ethanol.
Should not be stored at low pH for prolonged time
pH stability
2 - 13
2 - 13
3 - 9 recommended pH
3 - 13
Storage
2 to 25 °C in 20% ethanol
2 to 25 °C in 20% ethanol
2 to 25 °C in 20% ethanol
1. The median particle size of the cumulative volume distribution.
2. Dynamic binding capacity determined at 4-minutes residence time (0.25 ml/min in 1 ml column) in 20 mM Na-citrate, 60 mM NaCl, pH 4.0.
3. Dynamic binding capacity determined at 4-minutes residence time (0.25 ml/min in 1 ml column) in 50 mM Tris-HCl, 50 mM NaCl,pH 8.0.
4. Maximum flow rate for aqueous buffers at 20 °C. Decrease the maximum flow rate if the liquid has a higher viscosity. Higher viscosities can be caused by low temperature
(use half of the maximum flow rate for 20% ethanol).

IN 45 100 010 •AA •12 / 12
Related Products
Product name
Pack size1
Article number
Prepacked columns
BabyBio Dsalt 1 ml
1 x 1 ml
45 360 101
BabyBio Dsalt 5 ml
1 x 5 ml
45 360 105
OptioBio 40S 10x100
1 x 7.9 ml
55 420 011
OptioBio 40Q 10x100
1 x 7.9 ml
55 410 011
Bulk resins
WorkBeads 40S
25 ml
40 200 001
WorkBeads 40Q
25 ml
40 100 001
WorkBeads 40 DEAE
25 ml
40 150 001
Accessories
Column plug male 1/16”
10
70 100 010
Column cap female 1/16”
10
70 100 020
1. Other pack sizes can be found in the complete product list on www.bio-works.com
Ordering information
Product name
Pack size
Article number
BabyBio S 1 ml
1 x 1 ml
2 x 1 ml
5 x 1 ml
10 x 1 ml
45 200 101
45 200 102
45 200 103
45 200 104
BabyBio S 5 ml
1 x 5 ml
2 x 5 ml
5 x 5 ml
10 x 5 ml
45 200 105
45 200 106
45 200 107
45 200 108
BabyBio Q 1 ml
1 x 1 ml
2 x 1 ml
5 x 1 ml
10 x 1 ml
45 100 101
45 100 102
45 100 103
45 100 104
BabyBio Q 5 ml
1 x 5 ml
2 x 5 ml
5 x 5 ml
10 x 5 ml
45 100 105
45 100 106
45 100 107
45 100 108
BabyBio DEAE 1 ml
1 x 1 ml
2 x 1 ml
5 x 1 ml
10 x 1 ml
45 150 101
45 150 102
45 150 103
45 150 104
BabyBio DEAE 5 ml
1 x 5 ml
2 x 5 ml
5 x 5 ml
10 x 5 ml
45 150 105
45 150 106
45 150 107
45 150 108
For more information about local distributors and products please visit www.bio-works.com or contact us at
Bio-Works
Virdings allé 18
754 50 Uppsala
Sweden
This manual suits for next models
2
Table of contents
Popular Laboratory Equipment manuals by other brands

Master
Master MWC Series Installation and operation manual

Epson
Epson ELPDC02 High Resolution Document Imager - High Resolution Document... manual

Cole Parmer
Cole Parmer Stuart SC6PLUS quick start guide

Porkka
Porkka FESTIVO MED XW70L Additional manual

ADInstruments
ADInstruments PowerLab owner's manual

Elmi
Elmi CM-50 user manual

Hach
Hach Dr Lange LCK 529 Programming instructions

IKA
IKA ICC control operating instructions

Agilent Technologies
Agilent Technologies 1290 Infinity Flexible Cube user manual

Qiagen
Qiagen Hybrid Capture System Multi-Specimen Tube Vortexer... user manual

Tuttnauer
Tuttnauer 3840 ELV Operation & maintenance manual

Agilent Technologies
Agilent Technologies Bravo Platform user guide





