BioIntelliSense BioButton BIOST03020 User manual

Version: BBN-LBL-IFU-FULL Rev. 0.5 1
BioButtonTM Instructions for Use
AUGUST 2020
Indications for Use
The BioButtonTM system is a remote patient
monitoring system that is capable of, through a
wearable sensor device, continuously obtaining
physiological data from patients in both healthcare
settings and in-home settings for up to 90 days.
The physiological data collected by the
BioButton can include:
• Heart rate at rest
• Respiratory rate at rest
• Skin temperature
There are other non-medical data
to be collected and reported by the
device, including:
• Activity Level
• Body position
• Sleep Tracking
• Contact Tracing
The device is capable of remote transferring of
obtained physiological data from the patient’s
location to clinicians patients for storage,
analysis, and review.
The device is intended for use on general care
patients who are 18 years of age or older as
a general patient monitor, to provide
physiological information.
The data from the BioButton System is intended
for use by healthcare professionals as an aid to
diagnosis and treatment.
The device is not intended for use on critical
care patients.
Table of Contents
1Indications for Use
2BioButton Overview
2Package Contents
3Warnings and Precautions
4Use Instructions
5Replace Your Adhesive
5Troubleshooting and FAQs
6Safety and Regulatory Information
8Technical Specifications
9Guidance and Declaration
IN CASE OF EMERGENCY, CALL 911 IMMEDIATELY
Our support line is not for medical emergencies. If you believe you have an emergency, call 911.

Version: BBN-LBL-IFU-FULL Rev. 0.5 2
BioButton Overview
The BioButton wearable device is made of medical
grade silicone and is adhered with a double-sided
fabric adhesive.
Package Contents
• BioButton Device
• Fabric Skirt Adhesives
• Instructions for Use

Version: BBN-LBL-IFU-FULL Rev. 0.5 3
Warnings and Precautions
• Remove the BioButton prior to any defibrillation events. Clinical validation has not been performed for
persons who have a defibrillator or pacemaker device.
• Keep the device away from children and pets. The BioButton device may be a choking hazard and may be
harmful if swallowed.
• Press the BioButton’s button regularly to verify that it is working.
DO NOT wear device over excessive body hair.
Excessive body hair should be trimmed using
only an electric trimmer, before application.
DO NOT place on broken skin including wounds,
sores, or abrasions.
DO NOT submerge the device in morethan 3 feet
of water or submerge for longer than 30 minutes
at a time. Prolonged exposure to water may
cause the device to loosen from the skin.
DO NOT continue wearing if severe discomfort or
irritation occurs.
DO NOT exert excessive force, drop, modify, or
attempt to take apart the device. Doing so may
cause malfunction or permanent damage.
DO NOT wear or use the BioButton during a
magnetic resonance imaging (MRI) procedure
or in a location where it will be exposed to strong
electromagnetic forces.

Version: BBN-LBL-IFU-FULL Rev. 0.5 4
Use Instructions
BEFORE YOU START
a. Download and install the BioMobileTM app for iOS or Android phones.
We support iOS 13+ and Android 9+
b. Set up BioHubTM unit by following the instructions in the BioHub box.
DO NOT proceed until BioMobile app or BioHub is set up.
GET STARTED
1. Press and hold the button for 2 SECONDS. Proceed when the LIGHT blinks repeatedly.
2. Locate area on UPPER LEFT CHEST, two inches below collar bone.
3. TRIM ANY BODY HAIR using only an electric trimmer and CLEANSE AREA with a warm, damp cloth.
4. Find adhesive. Peel backing from DEVICE SIDE of adhesive.
5. Place the BioButton ON the exposed adhesive. Turn over and REMOVE remaining adhesive backing.
6. ADHERE BioBioButton to chest. Apply pressure for 15 seconds
You’re done!
LIGHT PATTERN MEANINGS
Press the BioButton’s button and confirm the light blinks 5 times.
LIGHTS MEANING
4 regular blinks Actively monitoring
5 rapid blinks Worn for maximum wear time (up to 90 days)
10 rapid blinks (repeated 4 times) Not monitoring, connect to BioMobile app or
BioHub to restart monitoring
Solid light or no light Error detected, contact support immediately
Version: BBN-LBL-IFU-FULL Rev. 0.5 5
Replace Your Adhesive
• When no longer sticky.
• If you experience redness or irritation in the placement area.
REMOVE adhesive from bottom of device. Follow steps 4 through 6 to put on a new adhesive and
reapply BioButton.
When replacing the adhesive, it is advised to apply the device to a dierent location within the placement area.
Troubleshooting and FAQs
Can I shower or exercise with my device?
Yes, the BioButton is water resistant and can be worn during showers and exercise. Do not apply any deodorant
or lotion to the placement areas as it will reduce adhesion of the device to the skin.
Can I swim or bathe with the BioButton?
Yes, the BioButton is water resistant and will continue working as long as it is not submerged more than 3 feet
or kept underwater for longer than 30 minutes at a time. Prolonged exposure to water may cause the device to
loosen from the skin.
I’m experiencing some skin irritation, what should I do?
Minor skin irritation and itching may occur while wearing the BioButton. If a severe reaction develops (i.e. hives
or blisters), discontinue wearing and immediately contact your physician.
How long should I wear my device?
Please wear your device for the entire monitoring period period, but no longer than 90 days. Each adhesive
may be worn for up to 7 continuous days. You may replace the adhesive more often, as needed, to ensure good
contact is maintained with the skin.
How do I know my device is working?
Press and release BioButton’s button. The device light will blink 4 times. If the device does not blink or blinks
more than 4 times, please immediately contact Customer Support (see front cover).
I’ve tried activating the device several times, and the light still won’t blink. What do I do?
Contact Customer Support immediately (see front cover). You may be instructed to return the device and may
receive a replacement kit if more data is needed for the monitoring period.

Version: BBN-LBL-IFU-FULL Rev. 0.5 6
Safety and Regulatory Information
FCC STATEMENT
Model: BIOST03020
FCC ID: 2ASE7- BIOST03020
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant
to Part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful
interference in a residential installation. This equipment generates uses and can radiate radio frequency energy
and, if not installed and used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a particular installation. If
this equipment does cause harmful interference to radio or television reception, which can be determined by
turning the equipment o and on, the user is encouraged to try to correct the interference by one or more of the
following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit dierent from that to which the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This
device may not cause harmful interference, and (2) this device must accept any interference received, including
interference that may cause undesired operation.
Changes or modifications not expressly approved by the party responsible for compliance could void the user’s
authority to operate the equipment.
This device complies with FCC Radiation Exposure limits set forth for an uncontrolled environment. This device
should be installed and operated with a minimum distance of 20 cm between the radiator and your body.
BioIntelliSense and BioButton are trademarks or registered trademarks of BioIntelliSense, Inc.
RESPONSIBLE PARTY:
BioIntelliSense, Inc.
570 El Camino Real #200
Redwood City, CA 94063
TERMS OF USE STATEMENT
NOTICE: Use of the BioIntelliSense Product(s) is subject to our
• Website and Product User Terms of Use at www.biointellisense.com/website-and-product-user-terms-of-use
• Website Privacy Policy at www.biointellisense.com/website-privacy-policy
• Product and Data-as-a-Service Privacy Policy at www.biointellisense.com/product-and-service-privacy-policy
By using the Product(s), you indicate you have read these terms and policies and that you agree to them,
including the limitations and disclaimers of liability. In particular, you understand and consent that use of the
Product(s) measures and records personal information about you, including vital sign and other physiologic
measurements. That information may include respiratory rate, heart rate, temperature, activity level, sleep
duration, body position, step count, gait analysis, coughing, sneezing and vomit frequency and other
symptomatic or biometric data. The Product(s) may also be configured to track and record proximity and
duration data in relation to other Product(s). You understand that the Product(s) do not render medical advice
or diagnose or prevent any specific disease, including any communicable disease or virus. If you have any
concerns about your health, including whether you have been exposed to or have contracted any disease or
virus, immediately contact your healthcare provider.
Version: BBN-LBL-IFU-FULL Rev. 0.5 7
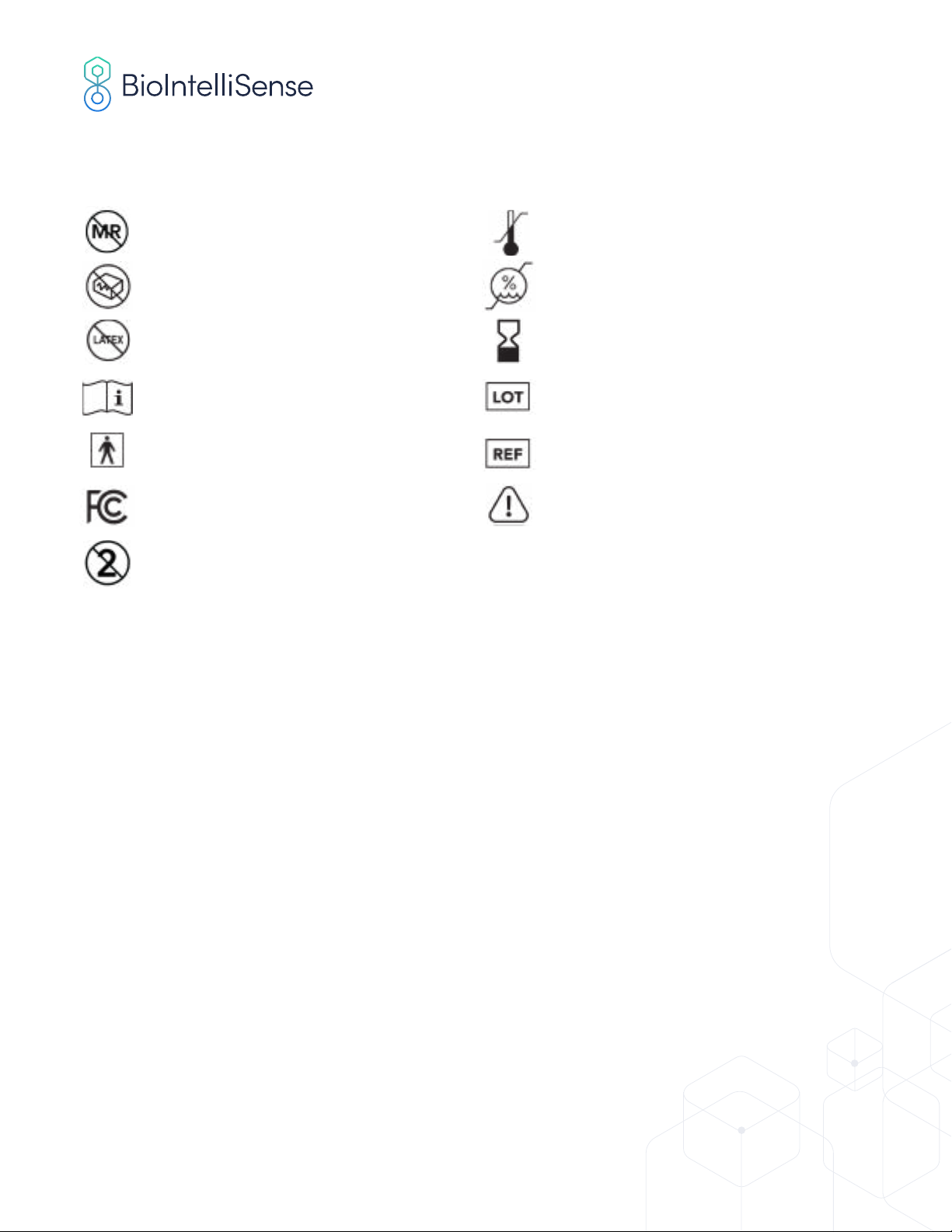
SYMBOL LIBRARY
MRI unsafe Temperature limitation
Don’t use if package is damaged Humidity limitation
Latex-free Apply by date
Consult with instructions for use Lot number
Type BF applied part Model number
FCC icon Warning
Single-use only

Version: BBN-LBL-IFU-FULL Rev. 0.5 8
Technical Specifications
Product Name BioButton
Model Number BIOST03020
Battery 140mAh, CR1632
Heart Rate*Range 40 to 125 beats per minute (<± 5 beats per minute)
Respiratory Rate*Range 10 to 30 breaths per minute
Skin Temperature Range 86°F to 107.6°F (30°C - 42°C)
Skin Temperature Accuracy < 96.4°F ± 0.5°F (< 35.8°C ± 0.3°C)
96.4°F to 98°F ± 0.3°F (35.8°C to 37°C ± 0.2°C)
98°F to 102°F ± 0.2°F (37°C to 39°C ± 0.1 °C)
102°F to 106°F ± 0.3°F (39°C to 41°C ± 0.2°C)
> 106°F ± 0.5°F (> 41°C ± 0.3°C)
Ambient Temperature 50°F to 110°F ± 1°F (10°C to 43°C)
STORAGE CONDITIONS
Temperature Range -4°F to 122°F (-20°C to 50°C)
Humidity Range 0 - 95% RH
OPERATING CONDITIONS
Temperature Range 32°F to 122°F (0°C to 50°C)
Humidity Range 0 - 95% RH
Atmospheric Pressure 70 - 102 kPa
Water Resistance IP47
COMMUNICATIONS
Communication Technology Bluetooth (BT4.2)
Distance Max. 10 meters (30 feet) line of sight
Radio Modulation GFSK Guassian frequency shift keying
Radio Frequency 2.4 – 2.5 GHz
Transmit Power 0dBm
Security AES-CTR 128 bit encryption (Advanced encryption standard counter mode)
* Measurements are taken at rest

Version: BBN-LBL-IFU-FULL Rev. 0.5 9
ELECTROMAGNETIC EMISSION
The BioButton sensor is intended for use in the electromagnetic environment specified below. The user of the
device shall ensure that the device is used in such an environment.
ELECTROMAGNETIC IMMUNITY
The BioButton sensor is intended for use in the electromagnetic environment specified below. The end user of
the device should assure that it is used in such an environment.
EMISSION TEST
METHOD
COMPLIANCE
LEVEL ELECTROMAGNETIC ENVIRONMENT & GUIDANCE
RF emissions
CISPR 11: 2009
+ AI:2010
GROUP 1
The BioButton sensor uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to cause
any interference in nearby electronic equipment.
RF emissions
CISPR 11: 2009
+ AI:2010
CLASS B
The BioButton sensor is suitable for use in all establishments,
including domestic establishments and those directly connected
to the public low-voltage power supply network that supplies
buildings used for domestic purposes.
Guidance and Declaration — Electromagnetic Compatibility
IMMUNITY TEST IEC 60601
TEST LEVEL
COMPLIANCE
LEVEL ELECTROMAGNETIC ENVIRONMENT GUIDANCE
Electrostatic
discharge (ESD) IEC
61000-4-2
± 8 kV contact
± 15 kV air
± 8 kV contact
± 15 kV air
Floors should be wood, concrete, or ceramic tile. If floors are
covered with synthetic material, the relative humidity should
be at least 30 %.
Power frequency
(50/60 Hz) magnetic
field IEC 61000-4-8
30 A/m 30 A/m
Power frequency magnetic fields should be at levels
characteristic of a typical location in a typical commercial or
hospital environment.
Radiated RF IEC
61000-4-3
10 V/m
80 MHz to 2.7
GHz
10 V/m
Portable and mobile RF communications equipment should
be used no closer to any part of the BioButton sensor
than recommended to the frequency of the transmitter.
Recommended separation distance:
d = 1.2 √P 80 MHz to 800 MHz
d = 2.3 √P 800 MHz to 2.7 GHz
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters, as determined by
an electromagnetic site survey(a) should be less than the
compliance level in each frequency range(b).
Interference may occur in the vicinity of equipment marked
with the following symbol:
Version: BBN-LBL-IFU-FULL Rev. 0.5 10
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is aected by
absorption and reflection from structures, objects, and people.
(a) Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones
and land mobile radio, AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic
site survey should be considered. If the measured field strength in the location in which the BioButton is
used exceeds the applicable RF compliance level above, the BioButton should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary, such as
reorienting or relocating the BioButton.
(b) Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
IMMUNITY TO RF WIRELESS COMMUNICATIONS EQUIPMENT
TEST
FREQUENCY
MHZ
BANDA
MHZ SERVICESERVICEAAMODULATIONBMAXIMUM
POWER W
DISTANCE
M
IMMUNITY TEST
LEVEL V/M
385 380390 TETRA 400 Pulse modulationb18
Hz 1.8 0.3 27
450 430470 GMRS 460,
FRS 460
FMc
± 5 kHz deviation
1 kHz sine
2 0.3 28
710, 745,
780 704 787 LTE Band 13, 7 Pulse modulationb
217 Hz 0.2 0.3 9
810, 870,
930 800 960
GSM 800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
Pulse
modulationb
18 Hz
2 0.3 28
1720, 1845,
1970 1700 1990
GSM 1800;
CDMA 1900;
GSM 1900;
DECT;
LTE Band 1, 3, 4,
25; UMTS
Pulse
modulationb
217 Hz
2 0.3 28
2450 24002570
Bluetooth, WLAN,
802.11 b/g/n, RFID
2450, LTE Band 7a
Pulse
modulationb
217 Hz
2 0.3 28
5240, 5500,
5785 5100 5800 WLAN 802.11 a/n Pulse
modulationb
217 Hz
0.2 0.3 9
aFor some services, only the uplink frequencies are included.
bThe carrier shall be modulated using a 50 % duty cycle square wave signal.
cAs an alternative to FM modulation, 50% pulse modulation at 18 Hz may be used because while it does not represent actual modulation, it would be worst case.

Version: BBN-LBL-IFU-FULL Rev. 0.5 11
RECOMMENDED SEPARATION DISTANCE BETWEEN PORTABLE AND MOBILE RF
COMMUNICATIONS EQUIPMENT AND BIOBUTTON
The BioButton is intended for use in the electromagnetic environment in which radiated RF disturbances
are controlled. The end user of the device can help prevent electromagnetic interference by maintaining
a minimum distance between portable and mobile RF communications equipment (transmitters) and
the BioButton sensor as recommended below, according to the maximum output power of the
communications equipment.
Contact Us
For non-urgent support or questions about our product, please call 888.908.8804
MANUFACTURED BY
BioIntelliSense, Inc.
570 El Camino Real #200
Redwood City, CA 94063
RATED MAXIMUM OUTPUT
POWER OF TRANSMITTER
W
SEPARATION DISTANCE ACCORDING TO FREQUENCY OF
TRANSMITTER M
80 MHZ TO 800 MHZ
D = 12 √P
800 MHZ TO 27 GHZ
D = 23 √P
0.01 012 0.23
0.1 038 0.73
1 12 2.3
10 38 7.3
100 12 23
Table of contents