Carolina 3M Petrifilm ColiformCount Plates User manual

82-4030
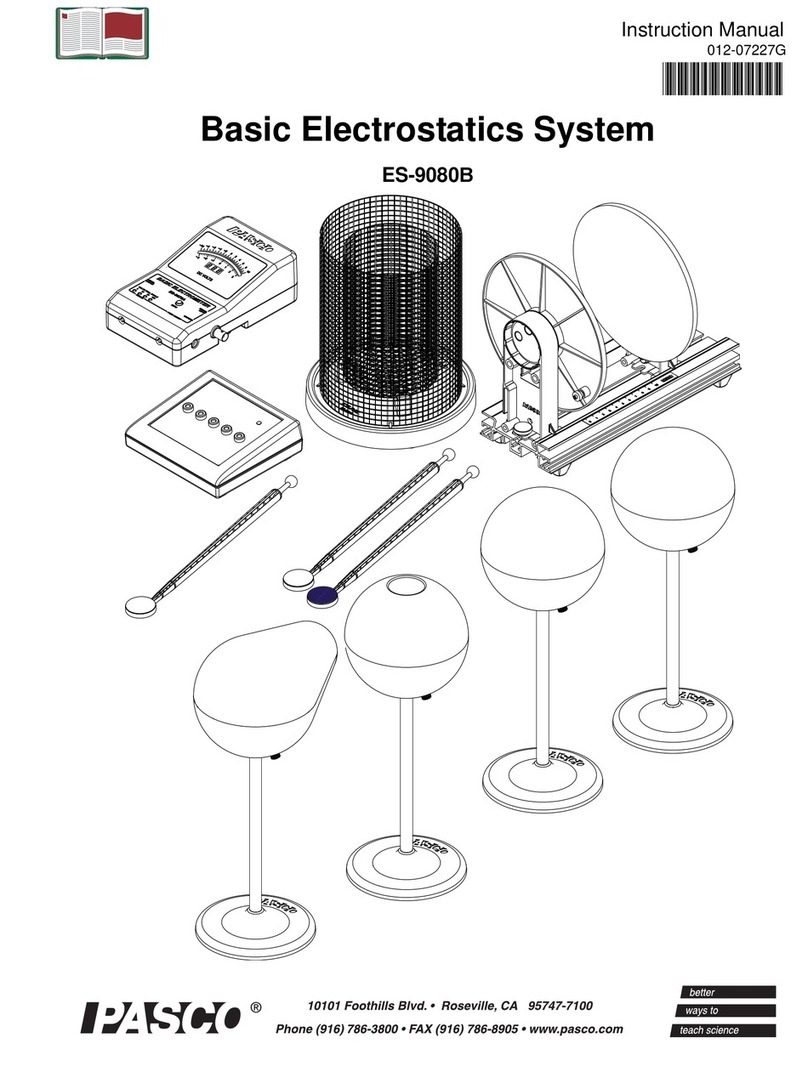
3M™ Petrifilm™Coliform
Count Plates
Instruction Manual
CAROLINA®
Teamed with Teachers

Description
The 3M™ Petrifilm™ Coliform Count (CC) Plate is a ready-made culture
medium system designed to facilitate counting coliforms. Petrifilm CC Plates
contain nutrients, a cold-water-soluble gelling agent, and an indicator dye.
Petrifilm plates are prepared on a grid background to facilitate counting
colonies.
Petrifilm CC plates are designed to allow organisms to grow in an oxygen-
limited environment. Their system of nutrients and dyes enables the user to
identify colonies formed by coliforms. For more information, please see
Interpretation. Petrifilm plates can be used in a variety of ways:
•Petrifilm plates can be hydrated with a bacterial culture, or a dilution of a
culture, for counting the viable organisms present. See Method A.
•Petrifilm plates can be hydrated first with water or buffer, then inoculated
by swabbing, streaking, or touching to surfaces. See Method B.
•Antibiotics can be added to the hydration fluid to select for resistant
organisms in Method A or B.
•Cells can be removed from colonies growing on Petrifilm plates and used
to inoculate additional cultures or for staining.
•Experimental results on Petrifilm plates can be saved for future reference
by scanning the plates on a standard computer-linked scanner. Do not
open the plates for scanning.
Petrifilm plates were developed for use in the food and beverage industry.
They have been certified for official analyses in many countries. For more
information about these applications, see the 3M page on the World Wide
Web at www.3m.com.
©2000 Carolina Biological Supply Company Printed in USA
2

3
Storage
Refrigerate unopened packages at ≤8˚C (≤46˚F). Use before expiration date
on package.
To seal an opened package, fold the end over and tape it shut (fig. 1).
Figure 1
Keep resealed packages at room temperature and less than 50% relative
humidity. Do not refrigerate opened packages. Use plates from the opened
package within one month after opening.
Directions for Use
Use sterile technique when handling Petrifilm plates. Disinfect the work area
by wiping thoroughly with alcohol or other disinfectant before and after use.
Used Petrifilm plates may contain viable organisms. Do not open the plates
unnecessarily. The procedures in this instruction booklet should be used for
educational purposes only.
Method A. Inoculation with liquid sample
Inoculate and spread each Petrifilm plate before going on to the next plate.
1. If a Petrifilm plate pack has been stored in the refrigerator, let the
package come to room temperature before opening it. This step
prevents condensation from forming inside the package.

2. Place the Petrifilm plate on a level surface, with the gridded side down.
Lift the top film (fig. 2).
Figure 2
3. With pipet perpendicular to the Petrifilm plate, place 1 mL of sample
onto the center of the bottom film (fig. 3). If necessary, samples can be
diluted with distilled water, liquid culture medium, or buffers with pH
between 6.6 and 7.2.* If antibiotics are to be added to the medium, add
them to the inoculating liquid at the working concentration.
Figure 3
4

5
4. Roll the top film onto the bottom film. Do not drop the top film down
(fig. 4).
Figure 4
5. With the flat side down (not the side with the circular ridge), place the
spreader on the top film over the inoculum (fig. 5).
Figure 5
6. Gently apply pressure on the spreader to distribute the inoculum over
a circular area. Do not twist or slide the spreader (fig. 6).
Figure 6
Ridge

7. Lift the spreader. Wait at least 1 minute for the gel to solidify (fig. 7).
Figure 7
8. Incubate plates with the gridded side down in stacks of up to 20 plates.
Incubation time and temperature will vary according to the application
and equipment available.
9. Colonies on Petrifilm plates can be counted on a standard colony
counter or other light source (fig. 8). Bacterial colonies on Petrifilm CC
plates are red because of the indicator dye in the medium. Refer to the
Interpretation section on pg. 8 and 9 for more details.
Figure 8
6

7
10. Colonies may be isolated for further study or to inoculate additional
cultures. Lift the top film and pick the colony from the gel. The medium
will adhere to the top film (fig. 9).
Figure 9
11. Disinfect before disposal. Petrifilm plates can be disinfected by
autoclaving or by soaking in 20% bleach for 1 hour. Then, they can be
placed in the trash. Alternatively, they can be taken to a facility such as
a hospital or given to a school nurse for disposal with other
biohazardous material.
*Do not use diluents that contain citrate or sodium thiosulfate because they
can inhibit growth on the Petrifilm plates. These substances are not found
in common microbiological media such as Luria broth or nutrient broth.
Method B. Hydrating and using as solid medium
1. Follow steps 1–6 from Method A, using 1 mL of distilled water, liquid
culture medium, or buffers with pH between 6.6 and 7.2 to hydrate the
Petrifilm plate.* If antibiotics are to be added to the medium, add them
to the hydration liquid at the working concentration.
2. Lift the spreader. Wait at least 2 hours for the gel to solidify.
3. Hydrated Petrifilm CC plates can be stored in a sealed bag in the
refrigerator for up to 7 days before use.

4. To inoculate the medium, lift the top film. The circular gel area will adhere
to the top film (fig. 10). Tape the Petrifilm plate to a flat surface in the open
position for streaking. Streak with a sterile inoculating loop more gently
and with less pressure than you would use on a standard agar plate.
Figure 10
5. Incubate the plates with the gridded side down, in stacks of up to 20
plates.
Colonies may be isolated for further study or to inoculate additional
cultures. Lift the top film and pick the colony from the gel (See Method A,
step 10).
*Do not use diluents that contain citrate or sodium thiosulfate. These
substances are not found in common microbiological media such as Luria
broth or nutrient broth.
Interpretation
The U.S. Food and Drug Administration Bacteriological Analytical Manual
defines coliforms as gram-negative rods that produce acid and gas from
lactose fermentation. Petrifilm CC Plates contain lactose, and the top film
traps gas produced by coliform growing on the plates. Coliform colonies
are identified by the gas bubbles associated with them. All bacterial
colonies on Petrifilm CC Plates are red because of an indicator dye in the
medium. The red color helps to distinguish them from dust particles or
other environmental contaminants.
Sometimes gas bubbles are trapped when the inoculate is spread on
Petrifilm plates. These bubbles are irregularly shaped and not associated
with a bacterial colony, while gas bubbles produced by lactose-fermenting
bacteria are associated with a colony (see fig. 11.)
8

9
Sample Plate
Figure 12.
Figure 11. Gas bubbles produced by colonies
Gas bubble not associated
with a colony

CarolinaBiologicalSupplyCompany
2700 York Road, Burlington, North Carolina 27215
CB162760002
To order call:
1-800-334-5551 (US and Canada)
336-584-0381 (International)
For technical help call:
1-800-227-1150
www.carolina.com
Table of contents