Coag-Sense®Prothrombin Time (PT)/INR Monitoring System Self-Test User’s Manual
Page 4 For In Vitro Diagnostic Use CoaguSense, Inc.
Table of Contents
Contacting CoaguSense......................................................................3
Table of Contents.................................................................................4
1. About This Manual.....................................................................5
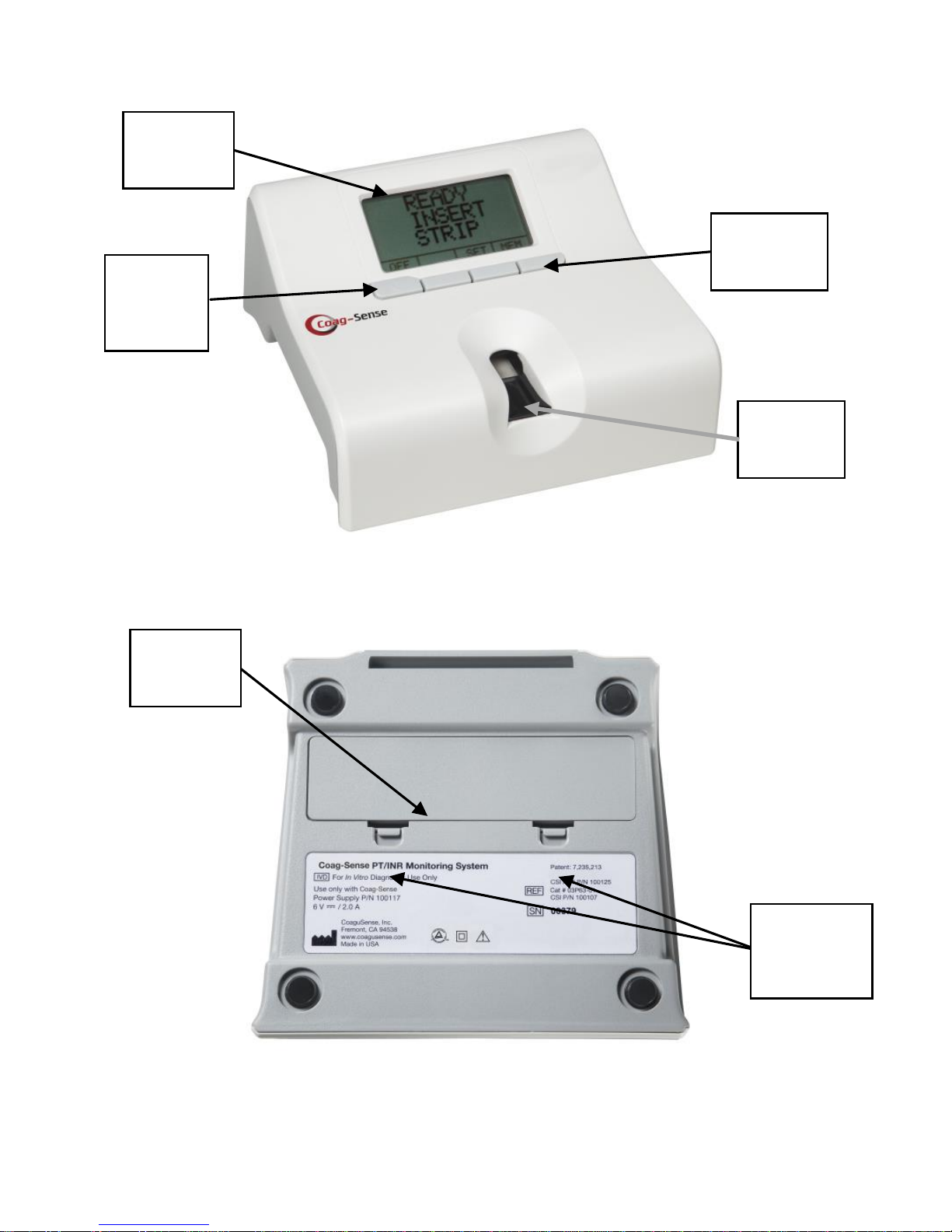
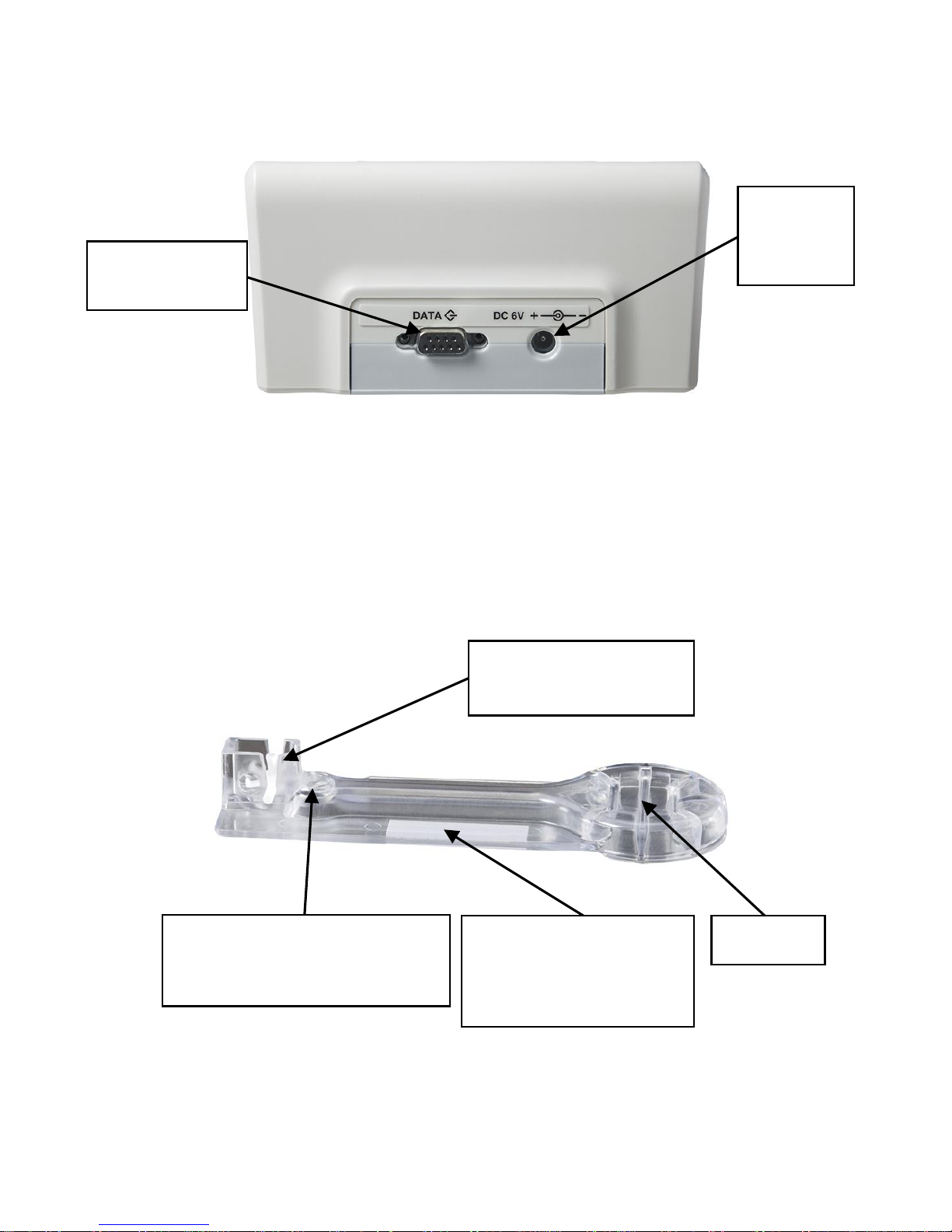
2. System Description....................................................................6
3. Reordering Information ............................................................10
4. Warnings and Precautions.......................................................10
5. Hazards and Symbols..............................................................15
Directions for Use ..............................................................................16
6. Operating Conditions ...............................................................16
7. Power On and Off ....................................................................17
8. Setting the Time and Date .......................................................17
9. Performing a Control Test........................................................19
10. Performing a PT Test...............................................................24
11. Collecting a Fingerstick Sample...............................................29
Sample Collection and Transfer Methods............Error! Bookmark not
defined.
12. Reviewing the Memory.............................................................34
13. Control Strips ...........................................................................35
14. Replacing the Batteries............................................................35
15. Cleaning the Meter...................................................................38
16. Troubleshooting .......................................................................39
17. Performance Characteristics....................................................46
18. Meter Specifications.................................................................47
19. Warranty ..................................................................................48
20. Index........................................................................................50