CoaguSense Coag-Sense User manual

Prothrombin Time (PT)/INR
Monitoring System
Coag-Sense®
Patient Self-Testing
User Manual
PST

No part of this publication may be reproduced, transmitted, transcribed,
stored in a retrieval system or translated into any language or computer
language, in any form or by any means, including, but not limited to, electronic,
magnetic, optical, chemical, manual, or otherwise without written permission
of CoaguSense, Inc. CoaguSense, Inc. has made every reasonable effort
to ensure that all the information contained in this manual is correct at the
time of printing. However, CoaguSense, Inc. reserves the right to make any
changes necessary without notice as part of ongoing product development.
If you have any questions or concerns with the Coag-Sense®Prothrombin
Time (PT)/INR Monitoring System, please contact CoaguSense, Inc.
Technical Support:
CoaguSense, Inc.
48377 Fremont Blvd., Ste 113
Fremont, CA 94538, U.S.A.
Technical Support: 1-866-903-0890
Email: [email protected]
© 2023 CoaguSense, Inc. All rights reserved.
Coag-Sense is a trademark of CoaguSense, Inc.

Table of Contents
1. Introduction ............................................................................... 1
2. System Description ..................................................................... 3
3. Meter Specications .................................................................. 11
4. Performance Characteristics ..................................................... 13
5. Warnings and Precautions ........................................................ 14
6. Hazards and Symbols ............................................................... 18
Directions for Use ........................................................................... 20
7. Meter Setup............................................................................... 20
8. Performing a Control Test ......................................................... 28
9. Collecting a Fingerstick Sample ................................................ 34
10. Performing a PT Test ................................................................ 37
11. Reviewing the Memory.............................................................. 42
12. Printing ...................................................................................... 44
13. Network Connectivity and Security............................................ 46
14. Bluetooth App Security .............................................................. 46
15. Meter Software Update ............................................................. 47
16. Battery ...................................................................................... 48
17. Cleaning and Disinfecting the Meter ......................................... 49
18. Troubleshooting ........................................................................ 51
19. Warranty ................................................................................... 59
20. Reordering Information ............................................................. 61
21. EMC Tables .............................................................................. 62
22. Index ......................................................................................... 64

The Coag-Sense®Prothrombin Time (PT)/INR
Monitoring System for Patient Self-Testing is intended to
be used by a single person and should not be shared.

Page 1
1. Introduction
Coag-Sense®Prothrombin Time (PT)/INR Monitoring System
Intended Use
For self-test users, the Coag-Sense®Prothrombin Time (PT)/INR Monitoring
System is an in vitro diagnostic device that provides quantitative prothrombin
time (PT) results, expressed in seconds and International Normalized Ratio
(INR) units. It uses fresh capillary whole blood.
The device is intended for use by properly selected and suitably trained
patients or their caregivers on the order of the treating physician to monitor
patients who are on anticoagulation therapy. Patients should be stabilized on
warfarin-type (coumarin) anticoagulation therapy prior to self- testing. The
device is not intended to be used for screening purposes.
Importance of PT/INR Monitoring
Blood-Clotting Time:
The rate at which blood clots is measured in units called International
Normalized Ratio (INR). It is very important for patients to stay within their
individual target INR range. If the INR is too low, the risk of blood clots
increases. If the INR is too high, the risk of hemorrhaging increases. The
patient’s physician will determine the most appropriate INR range for the
patient, depending upon the patient’s indication and how they respond to the
oral anticoagulants.
Anticoagulation Medication:
Oral anticoagulation medications, are typically prescribed to patients to
avoid unwanted clots. The patient’s blood clotting time must be monitored to
ensure that their dosage is correct.
Oral anti-coagulation medication is prescribed to patients with acute and
chronic conditions including, but not limited to: congestive heart failure, atrial
brillation, prosthetic heart valve, myocardial infarction, joint replacement,
deep vein thrombosis, pulmonary embolism, thrombotic stroke, coronary
artery disease, cancer and venous thromboembolism.

Page 2
Important Information Regarding This Manual
The purpose of the Coag-Sense®Prothrombin Time PT/INR Monitoring
System User Manual is to help you understand your Coag-Sense®PT/
INR System, its parts, and its intended function. It provides you with the
information you need to perform a PT test with the Coag-Sense®PT/INR
System.
The Coag-Sense®System should only be used with a doctor’s
prescription. Do not adjust your medication without talking to your
doctor or health care professional.
You must complete proper training on the Coag-Sense®PT/INR System
before you begin using the system. It is also important to read this entire User
Manual and the inserts that come with the disposable Coag-Sense®Test
Strips. This User Manual has different formats and symbols to distinguish
warnings, notes, and meter buttons.
WARNING: This indicates a warning or precaution.
Please read and understand all warnings and precautions.
They tell you about potential safety hazards and situations that
may cause injury. If you have any questions, please contact
CoaguSense, Inc. Technical Support at 1-866-903-0890.

Page 3
2. System Description
The Coag-Sense®Prothrombin Time PT/INR System is used for quantitative
measurement of INR (International Normalized Ratio) based on a
Prothrombin Time (PT) response to monitor the effect of therapy with vitamin
K antagonists like Coumadin®(warfarin). The system uses fresh, capillary
whole blood.

Page 4
Meter:
The meter has a TFT color LCD Touch Screen that shows results,
information, icons and results recalled from memory. To select an option,
gently click on the display button. There are three touch buttons, Cancel or
Previous Screen Button, Home Screen Button and View Menu Button
Screen for the Guided User Interface (GUI) operation. The Power ON/
OFF button is located on the right side of the meter. The NFC (Near Field
Communication) Tag Scanner is a built-in scanner that is used to scan the
NFC Tag containing the strip (Control and Test Strip) data. Strip Insertion
Area guides the test strip into the meter. Micro USB/Power Adapter Port
is a micro-USB port used to plug to the power adapter. Multipurpose USB
Port can be used to connect the meter to a) portable printer or other Coag-
Sense®System approved accessories. Ethernet Port is used to connect the
Ethernet cable for a wired connection, this port is provided with a port cover.
Reset Button (enclosed within the ethernet port cover) is used to reset
the meter in case of software or power-cycle issues.
The meter performs a self-check when it is rst powered ON and every
time a test strip is inserted. If there are any problems detected during self-
check, an error message is displayed on the touchscreen. Refer to the
“Troubleshooting” section of this manual or contact Technical Support for
assistance.
Test Strips:
A test strip is inserted and heated in the meter prior to sample application.
The strip contains a tiny wheel with spokes that pulls the sample into the
reaction well. The spokes quickly and completely mix the sample with the clot
initiating component of the test strip.
The PT time is determined from when the sample is drawn into the reaction
well of the test strip and detected by a beam of light until a clot forms and
interrupts the beam of light. The PT result is converted to an INR (International
Normalized Ratio) using the INR normalization data communicated by the
NFC Tag and subsequently stored in the meter. INR is a mathematical
correction of the PT result that adjusts for sensitivity differences among
different PT systems.

Page 5
Control Strips and Control Activation Solution
Quality control is an important part of PT testing to verify the integrity of the
performance characteristics of the testing system. The Coag-Sense®Meter
has been designed with multiple internal systems to ensure proper system
function. When powered ON, the meter runs an extensive self-check protocol
to ensure, for example, that operating temperature, timing functions, battery
level and optical and mechanical functions are within specication. There
are 2 Low and High Control Strips, and a Control Activation Solution shipped
with each test strip kit. Each control strip contains plasma which is generated
from a pool of normal donors where the Vitamin K dependent proteins are
removed and added back at different levels to represent the ‘Low’ and ‘High’
level ranges. Real plasma allows for a fully functional quality control test of
both the a) reagent’s ability to generate a clot and b) analyzer’s ability to
detect a clot. Control testing conrms the performance of the system and
should be completed immediately for each new lot of test strips received.
NFC Tag:
Near Field Communication (NFC) Tag is a micro data tag with antenna that
contains the required lot specic test strip kit information. Transfer of the lot
specic data to the meter can be accomplished through surface contact of
the matching NFC symbols. The NFC Tag communicates the unique data
for each lot of strips to the meter. The meter reads the data stored in the
NFC Tag and auto populates the relevant test strip information on the screen.
In the absence of NFC Tag, the user may manually enter the Lot and Barcode
number present on the strip packaging using the keypad on the touch screen.
A stylus with a rubber capacitive tip may be used to facilitate typing.
Handle
Mixing Wheel
and Reaction Well
Sample Application Area
(Light flashing when
ready for sample)

Page 6
Power Supply and Battery:
Coag-Sense®PT/INR Patient Self-Test System can be operated only with
the power adapter provided. The power adapter also serves as a charger. It
charges the built-in Lithium Polymer Battery. The battery life is shown on top
right corner of the meter.
Note: The battery is not user replaceable.
To save power, if left unattended for a set time (user congurable), the meter
will enter sleep mode. To power meter OFF, a manual press and hold of the
Power Button is required. The meter retains all results obtained up to that
point in its memory.
Coag-Sense®PT/INR Patient Self-Test System (Catalog #03P70-01) is
supplied with the following items:
Catalog Name QTY
Coag-Sense®PT/INR Meter 1
Coag-Sense®PT/INR System Self-Test User's Manual 1
Coag-Sense®PT/INR System Self-Test Quick Reference Guide 1
A/C Micro USB Power Supply 1
Sample Transfer Tubes 1pk
Single-Use, 21g Auto Safety Lancets 1pk
Carrying Case 1
Stylus Pen 1

Page 7
If you participate in a testing service, your service provider will provide you
with all the necessary testing components. If you run out of testing supplies,
please contact the service provider that gave you your meter for more
supplies. If you purchased a Coag-Sense®Test Strip Kit (Catalog# 03P56-
50) out-of-pocket, it will include the following items necessary to perform a
test:
Following are standard medical supplies that are required with each use and
may be supplied by your testing service provider:
21g Auto Safety Lancets, single use
Note: These materials are not provided with the PT/INR system. The Coag-
Sense®Test Strip Kit- 50 may be ordered from your meter distributor or home
testing service provider separately.
Item Description QTY
Patient Test Strips 50
Low Control Strips 2
High Control Strips 2
Control Strip Activation Solution 1
Sample Transfer Tubes 1pk
NFC Tag 1
Package Insert 1
Page 8
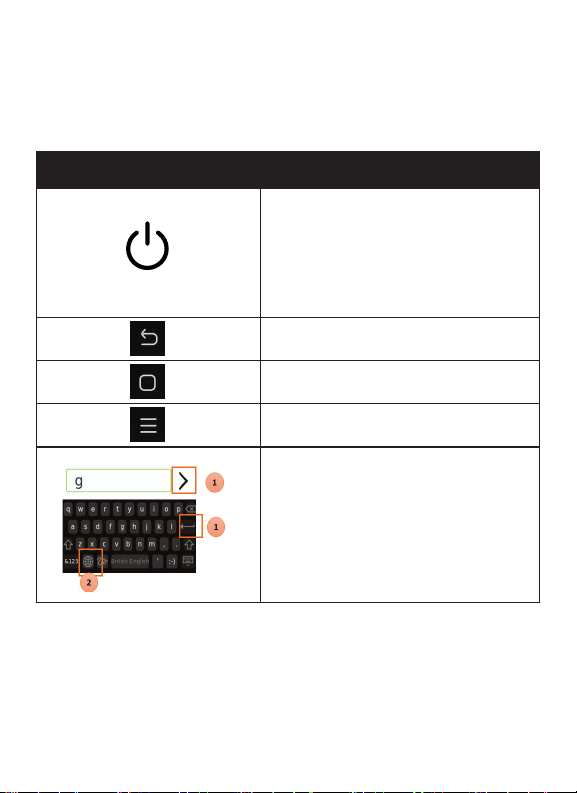
Overview of Buttons and Icons
The buttons and icons that appear during normal operation are shown here,
along with their respective meanings. Error messages and their description
are provided in ”Troubleshooting” section.
Buttons/Icons Meaning
Power ON/OFF
To power ON the meter, press and
hold Power Button. To Enter/Exit Sleep
Mode press the button once quickly and
press the button again and hold for few
seconds.
Cancel or return to
previous screen
Go to the home screen
View additional menu
Common Keypad Input
Is the input completion button. Returns
to previous screen when selected.
Change language button. Enables the
user to select keyboard language.

Page 9
Buttons/Icons Meaning
The Status Bar
1. Current Date/Time
2. Sound On/Off status
3. Alarm – The presence or absence of
set alarm
4. Bluetooth On/Off status
5. Wi-Fi On/Off Status
6. Battery status Icons on the
Touchscreen
The Icons on the Touch Screen
7. Back Icon - Go to previous screen
8. Home Icon - Go to home screen
when touched
9. Settings Icon - Go to settings screen
when touched
10. User Information Icon -
Go to user Information screen when
touched
11. Log-out Icon - Go to log-out pop-up
screen when touched.
Page 10
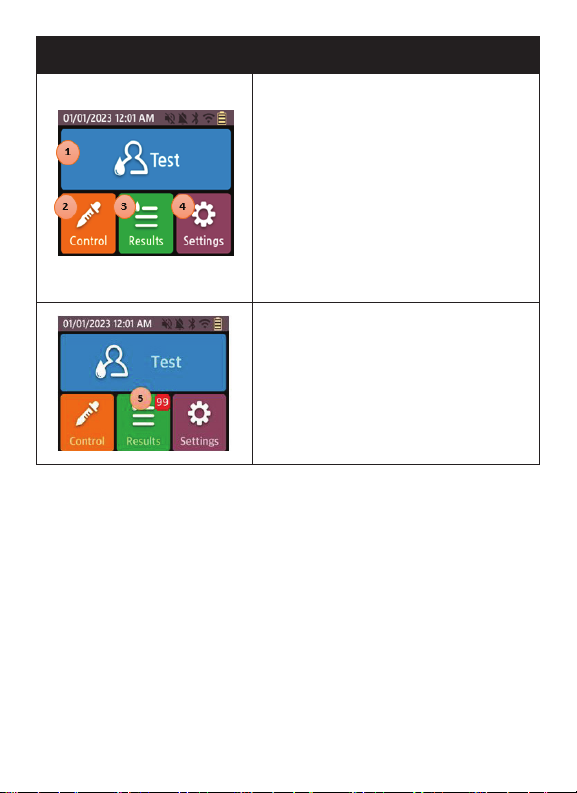
Buttons/Icons Meaning
Home Screen
1. Test Icon - Go forward to test screen
when touched.
2. Control Icon - Go forward to control
test screen when touched.
3. Results Icon - Go forward to result
screen when touched.
4. Settings Icon - Go forward to setup
screen when touched.
5. Results Icon - Displays the number
of results that have not been
synchronized.

Page 11
Operating Temperature 65°F to 90°F (18°C to 32°C)
Operating Humidity 10% to 90% (without condensation)
Storage Temperature 32°F to 122°F (0°C to 50°C)
Storage Humidity 20% to 80% (without condensation)
Altitude 10,000 ft (3,048 m) above sea level
Memory
Capable of storing up to:
• 2000 patient test results with date and time
• 500 control test results with date and time
• 1000 Operator accounts
Lithium Battery Rechargeable Lithium Polymer Battery
(3.7V, 2350mAh)
Battery Capacity Fully charged battery (6 hours of charging)
can run ~100 tests
Power Input 120V AC Adapter (Use with Coag-Sense®
Adapter Only)
AC Input 100-240V~, 50-60Hz, 0.5A
(Mains supply voltage uctuation: ±10%)
Power Output 5.0V, 2.0A
Pollution Degree 2
Overvoltage Category II
Use Circumstance Indoor only
Blood Sample Size 10-12 µL
Communication Port Micro and Standard USB
3. Meter Specications

Page 12
Size in mm
(Height x Width x Depth) 152 x 100 x 29.5
Weight in grams 315 grams
Equipment Classication
Class II with external power supply.
Internally powered when operated with
battery. IPX0 rating.
WARNING: Use the Coag-Sense®Meter along
with the provided Power Adapter only. Use only AC adapter
type UES12lCP-050200SPA (Manufacturer: Dongguan
Shilong Fuhua Electronics).

Page 13
4. Performance Characteristics
Expected Values:
Results are reported in INR units equivalent to the plasma reference method.
For PT testing, variations in the source of thromboplastin may cause some
differences in results between methods. It is recommended that the same
method be used to monitor the anticoagulation therapy over time.
Measuring Range:
INR 0.8 to 8.0 units
Normal Range: The following results represent a common normal range
for an individual in good health using the Coag-Sense®PT/INR Monitoring
System.
INR: 0.8 to 1.2 units
PT: 11.6 to 14.5 seconds

Page 14
5. Warnings and Precautions
• Patients taking Warfarin (Coumadin®) and other oral blood thinners
should consult with their healthcare provider before adjusting their
dosage.
• Patients should consult with their doctor for their appropriate INR
therapeutic range.
• Patients who have recently taken or are currently taking any type of
Heparin or Low Molecular Weight Heparin anticoagulant should not use
this test system and should consult their doctor.
• The system should also not be used to monitor patients on direct oral
anticoagulants (DOACs) including Factor Xa and Direct Thrombin
inhibitors.
Test Site and Blood Sample
• The Coag-Sense®PT/INR System is for in vitro diagnostic use only.
• The Coag-Sense®Meter will not produce a result if the test strip is past its
expiration date.
•The quality of the blood sample can affect PT test results. A blood
sample of poor quality can produce unreliable results. Read the section
on “Collecting a Fingerstick Sample” for more information.
•Blood samples must be applied to the test strip immediately after
collection or the blood begins to clot, causing unreliable results.
•The blood sample transferred to the test strip must be a minimum of 10
µL in volume. Low sample volume may cause an error message.
•Use only fresh ngerstick capillary blood for testing. The blood should
only come in contact with the Sample Transfer Tubes provided with the
Coag-Sense®PT/INR System. Other products may have anti-coagulant
agents on their surfaces and result in unreliable test results.
•Squeezing the ngerstick site excessively (milking) releases interstitial
“tissue layer” uid that can cause unreliable results.
•The ngerstick site should be washed with warm water and soap, and
then completely dried. The site must be clean of all hand oils/lotions and
foreign matter, which may cause unreliable results.

Page 15
• If Isopropyl Alcohol (IPA) wipes are used, wipe the ngerstick site
with a gauze pad and make sure the site is completely dry. If any
alcohol remains (or is re-introduced) on the nger, it may cause
unreliable results.
• Do not use wipes containing chlorhexidine gluconate, as it may produce
unreliable results.
• The quality of ngerstick and the sample delivery technique are important
to the test results. If there is a question about the sample or sample
collection, obtain a new strip, repeat the ngerstick on a different nger,
and test again.
• If you need to repeat a test, use a different nger for the ngerstick,
since blood may have started to clot on the rst nger, which may cause
unreliable results.
• If there is a bubble or an air pocket showing in the blood sample in
the collection tube, start the test over. Use a new ngerstick (using a
different nger and collection tube) or results may be unreliable.
Meter
• The meter has a built-in rechargeable lithium polymer battery (3.7 V,
2350 mAh).
• Use only the power adapter included with the Coag-Sense®System or
damage to the meter may result.
• The meter shall be in the position that it is easy to disconnect power.
• The meter is a delicate instrument, and should be handled with care.
Dropping or other mishandling may cause damage to the meter. If this
should occur, call Technical Support.
• Do not allow any liquids to spill on the meter. If this should occur, call
Technical Support.
• Do not put the meter in liquid. Do not allow liquids to get into any of the
connectors or plugs on the meter.
• Only use the method provided in this User Manual to clean the Coag-
Sense®PT/INR Meter. For cleaning purposes, please use a Healthcare
Bleach Germicidal Wipes containing Sodium Hypochlorite (Bleach) to
clean the exterior meter housing only. DO NOT SPRAY ANY LIQUIDS
DIRECTLY ONTO THE METER.

Page 16
• Do not move or touch the meter while it is running a test. Unreliable
results may occur.
• Do not pull the strip out during a test while the wheel is spinning. STOP
the test by pressing the cancel or back arrow. The display prompts you
to conrm test cancellation. The strip should be removed after conrming
test cancellation.
• Store and use the Coag-Sense®PT/INR System following the instructions
in this manual.
• This equipment is tested to meet the limits for medical devices, which are
designed to provide a reasonable protection against harmful interference
when the equipment is operated in a clinical or home environment. If not
installed and used in accordance with these instructions, it may cause
harmful interference to other devices in the vicinity. If this equipment does
cause harmful interference to other devices, which can be determined by
turning the equipment on and off, the user is encouraged to try to correct
the interference by one or more of the following measures:
• Reorient or relocate the receiving device.
• Increase the separation between the equipment.
• Connect the equipment to an outlet on a circuit different from that to
which the other devices are connected.
• Any equipment connected to the data port must be certied to IEC
60601-1. If you connect any equipment that is not recommended by
CoaguSense, Inc., you are responsible for meeting the requirements of
this standard.
• In the unlikely event of an electric power surge (i.e., severe static
discharge during a thunderstorm), when using the power adapter, the
display screen may go blank. If this occurs, unplug the power supply
from the back of your meter, wait 5 seconds and plug it back in. Normal
operation should return, but you may have to reset the time and date.
• DO NOT OPEN THE METER. Do not attempt to repair or modify
this meter. The Coag-Sense®Meter does not require any periodic
maintenance and there are no user serviceable parts inside. If you
have problems, please contact Technical Support. The Coag-Sense®
Prothrombin Time PT/INR Monitoring System needs special precautions
regarding EMC and needs to be put into service according to the EMC
information provided in this manual.
Other manuals for Coag-Sense
2
Table of contents
Other CoaguSense Measuring Instrument manuals
Popular Measuring Instrument manuals by other brands

Taylor
Taylor TTI Series quick start guide

Panasonic
Panasonic WJ-NX100/2E installation guide

SBC
SBC Saia PCD ALD1B5FS Assembly and operating instructions

Flowserve
Flowserve StarTalk DTM quick start guide

Amprobe
Amprobe AMB-45 instruction manual

Rohde & Schwarz
Rohde & Schwarz NRT 1080.9506.02/.62 operating manual
















