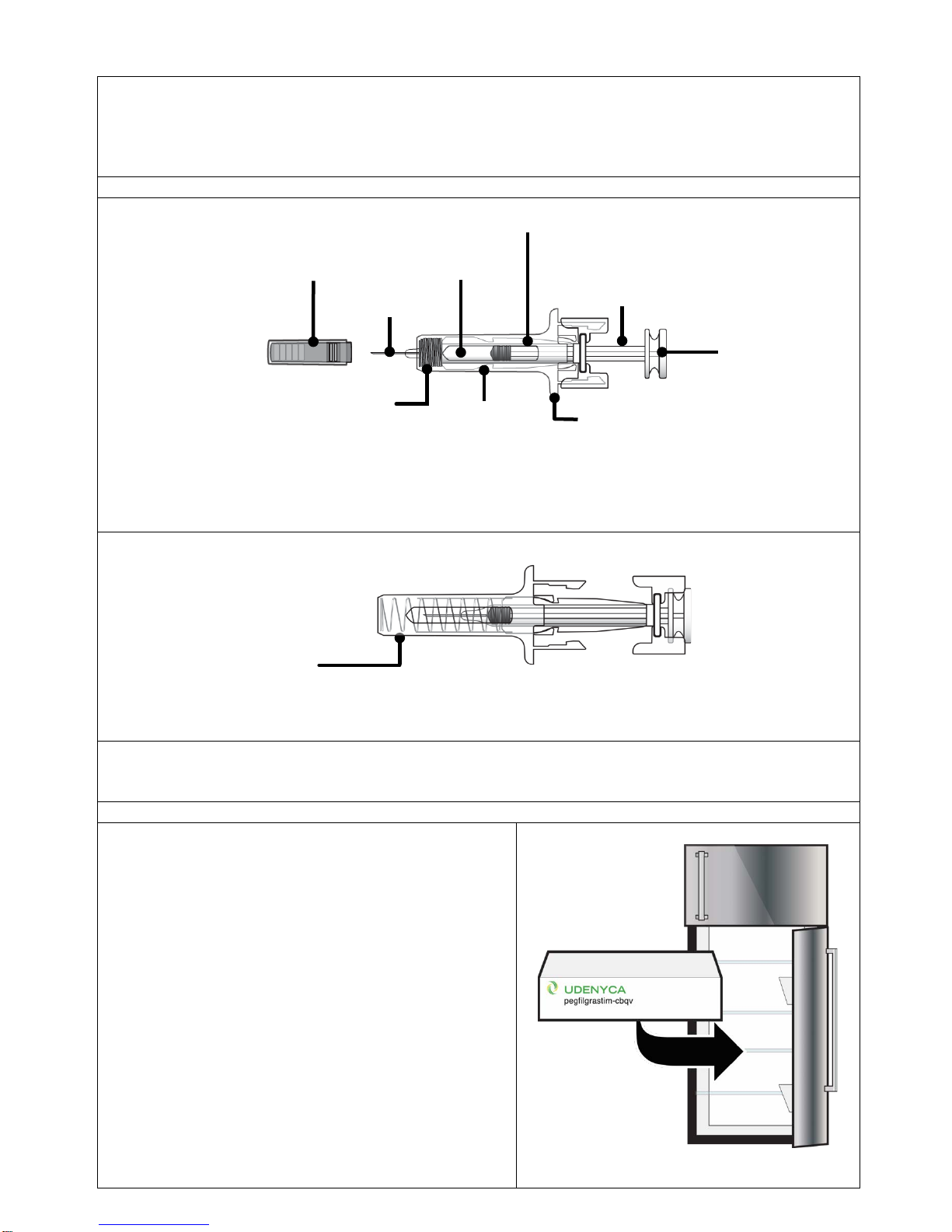
Select and clean injection site
5 - Select and clean the injection site
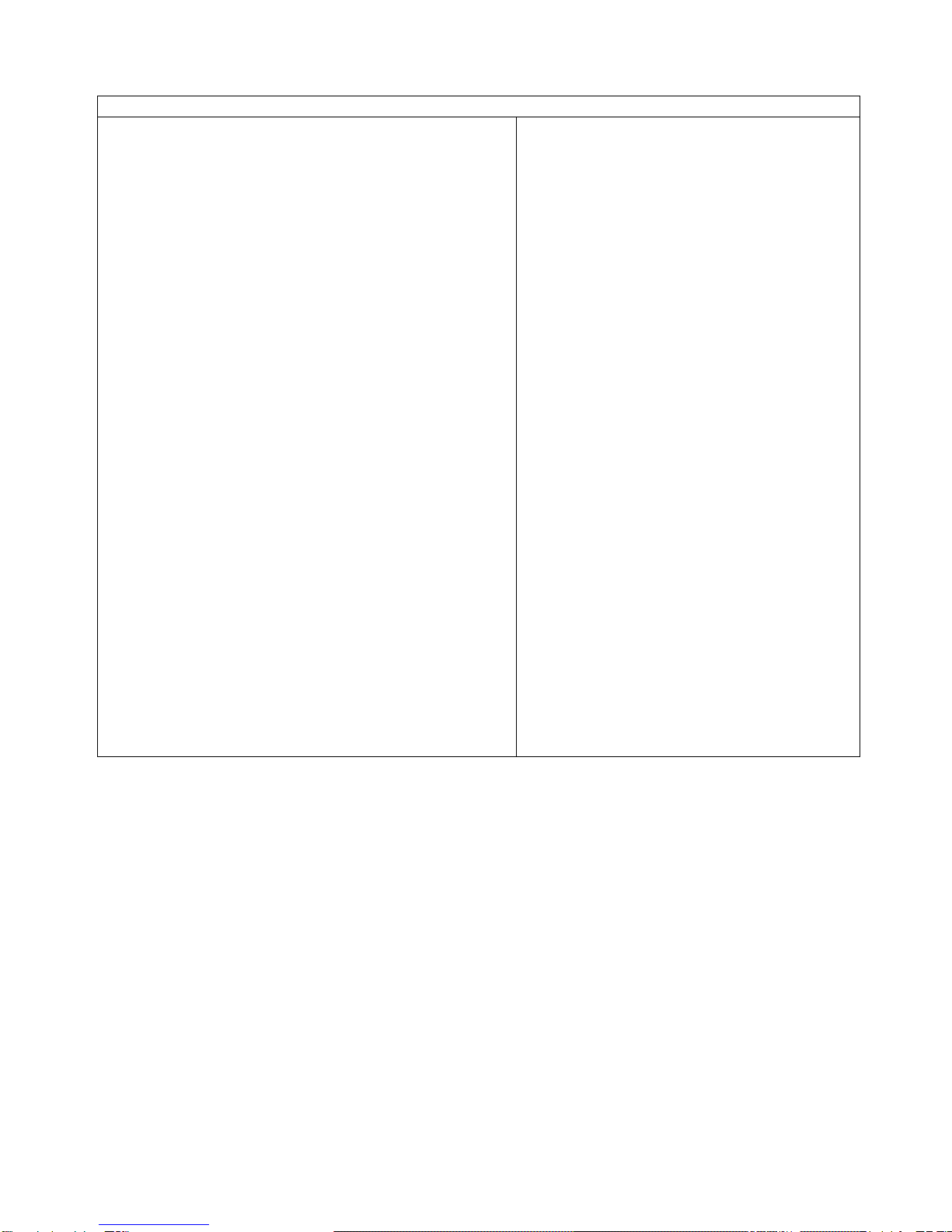
5A: Select the injection site. The recommended
injection sites for a subcutaneous injection are
the: (See Figure 10)
•Abdomen (except for a two-inch area
surrounding the navel)
•Thighs
•Back of upper arms (only if someone else is
giving you the injection)
•Upper outer area of the buttocks (only if
someone else is giving you the injection)
Do not inject into moles, scars, birthmarks, or
areas where the skin is tender, bruised, red, or
hard.
If you want to use the same injection site, make
sure it is not the same spot on the injection site
you used for a previous injection.
Figure 10
5B: Clean the injection site with an alcohol wipe.
(See Figure 11)
Do not touch this area again before injection.
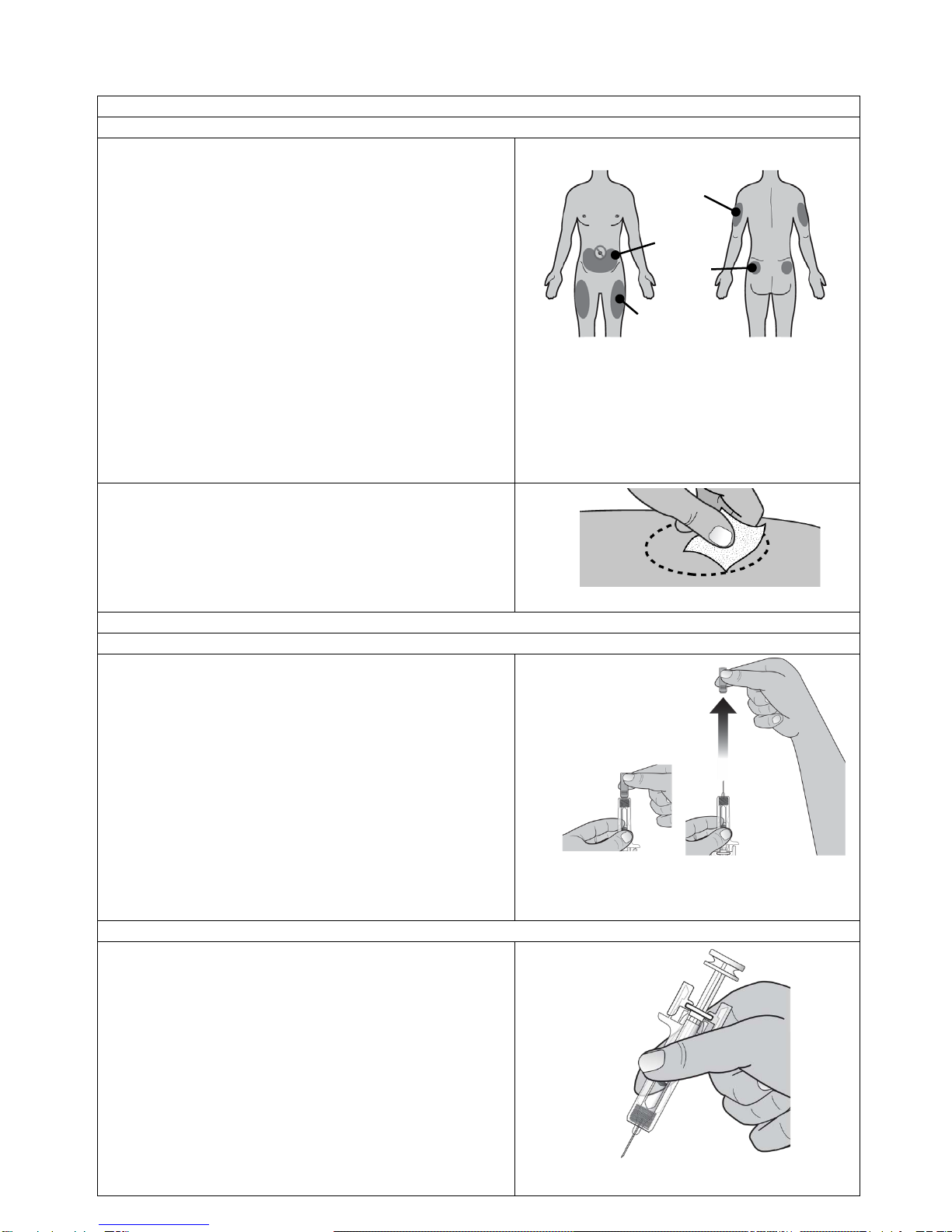
Remove the needle cap by pulling it straight off.
(See Figure 12)
•Do not remove the needle cap from the
prefilled syringe until you are ready to inject.
•Do not twist or bend the needle cap.
•Do not hold the prefilled syringe by the
plunger rod
•Do not put the needle cap back onto the
syringe. Dispose of (throw away) the needle
cap in your household trash
•Do not use the prefilled syringe if it has
been dropped with the needle cap removed.
Figure 12
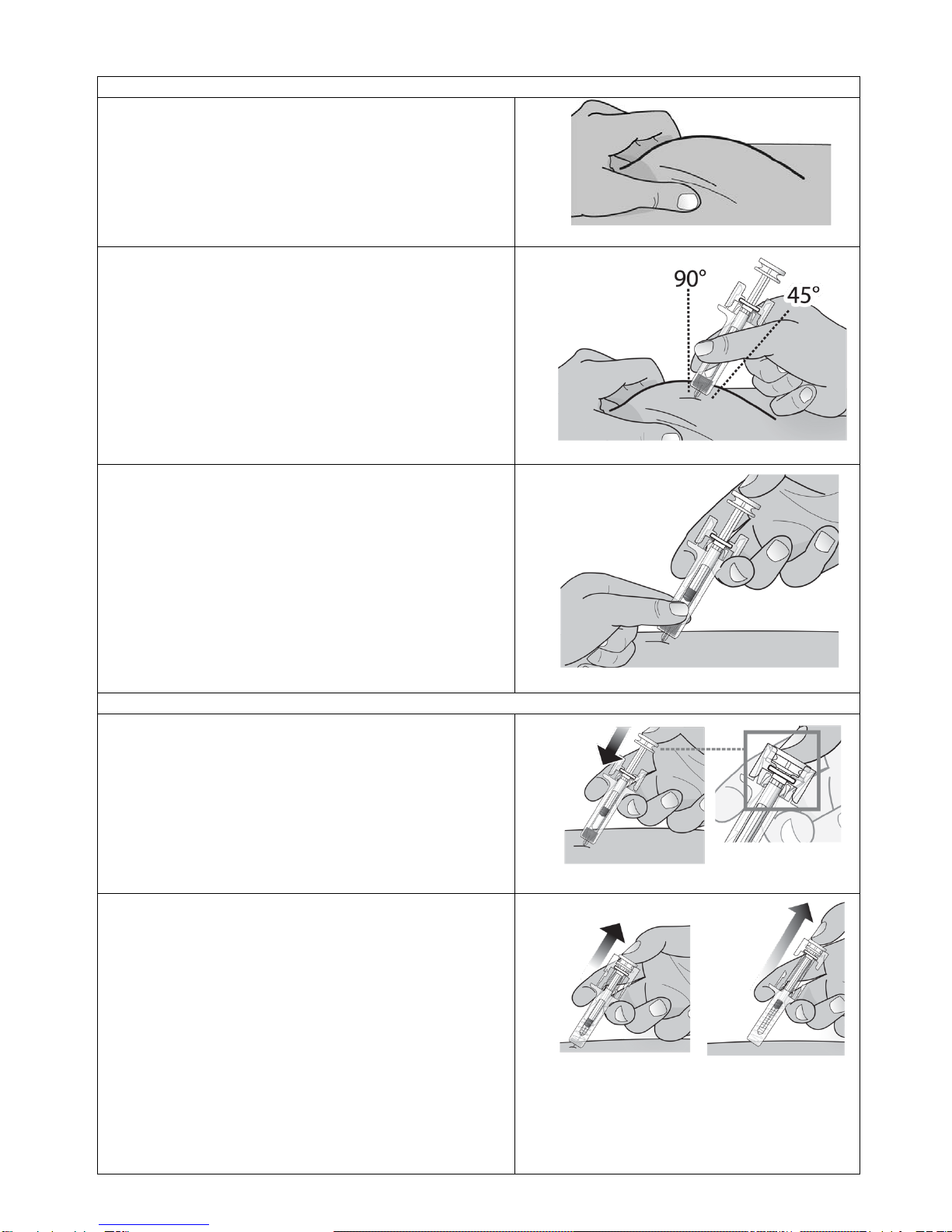
Grasp the body of the syringe like a dart (just
under the finger grips) with your thumb and index
fingers. (See Figure 13)
•Do not touch the plunger or grasp the
syringe above the finger grips.
outer