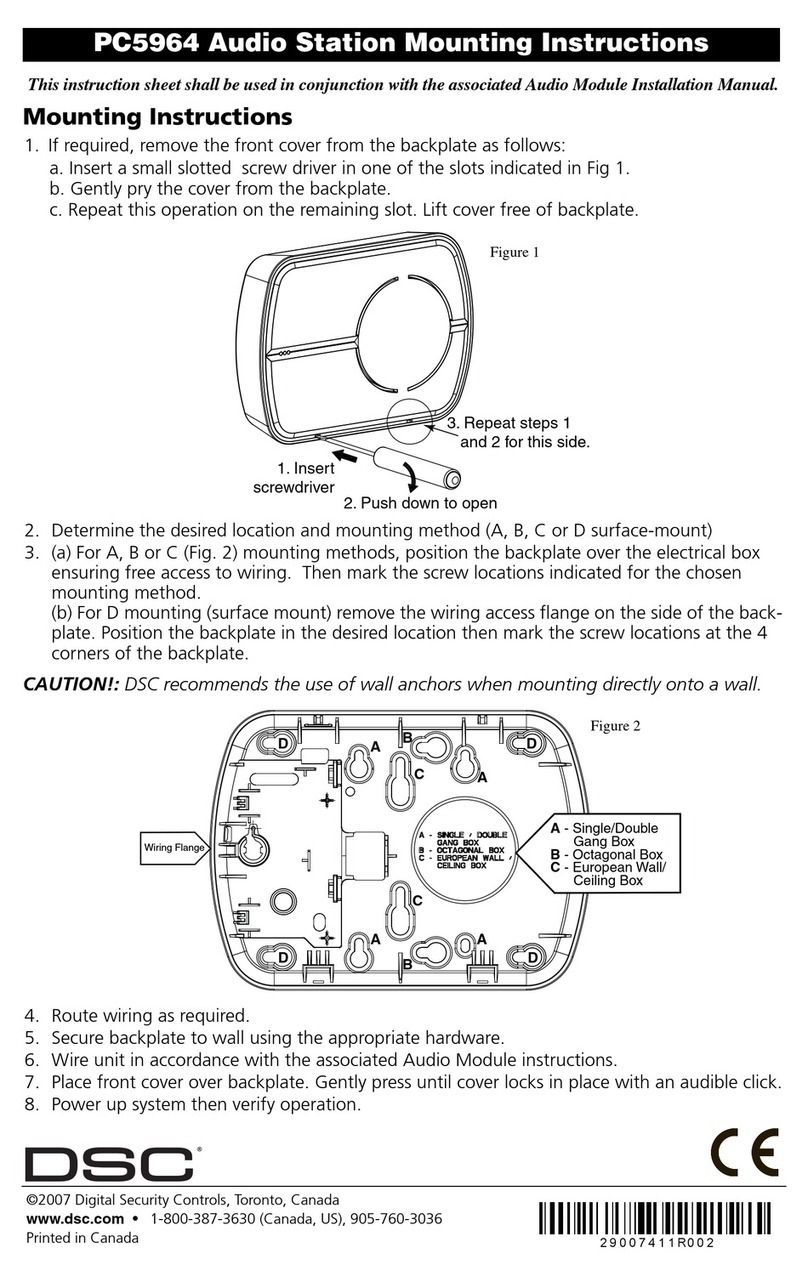
5Scope and general Information
Thank you for choosing this product.
The Cube Reader is a photometer, intended for the qualita-
tive, semi-quantitative or quantitative measurement of the
optical density of lines on test strips used in Lateral Flow As-
says (LFAs) / rapid tests.
The respective test-specific data is transmitted wirelessly be-
fore the measurement using RFID (Radio Frequency Identifi-
cation). Before each measurement, please ensure that the lot
number of the test matches that on the RFID tag.
The measurement results can be stored internally. In addition
to its own measurement ID (identification number of the
measurement), each measurement result contains the test
name, lot number, the name of the test manufacturer, as well
as the date and time of the measurement. The measurement
results can be read out via a special USB cable using the Cube