5
0473 90001Rev07.26.13
Radiation Safety:
X-rays are dangerous to both operator and others in the vicinity unless established safe
exposure procedures are strictly observed. Use the following safety measures to ensure safety
to the Patient and Operator. The useful and scattered beams can produce serious or fatal
bodily injuries to Patients and persons in the surrounding area if used by an unskilled operator.
Adequate precautions must always be taken to avoid or reduce exposure to the useful beam or
to scattered radiation. Operators are strongly urged to comply with the current
recommendations of the International Commission on Radiological Protection and, in the United
States, the US National Council for Radiological Protection.
Use the following measures to protect yourself and the patient from unintended exposure to
radiation. Anyone who is near the patient during test procedures must observe the following
precautions:
Maintain distance from exposed radiation source in accordance with the facility
survey or site plan and shielding designs, provided by a medical physicist. The
plan/survey will be created based off of Scatter Measurements provided in this
manual.
Keep exposure times to a minimum.
Patient must wear protective X-ray shielding items (lead apron, etc.) to protect
anatomical areas. We recommend all patients wear a protective shielding full wrap
apron. We recommend that patients less than 21 years old, small size patients
(under 100 pounds) and children also wear a gonad and ovarian front and back
protective shield. Sample shielding products, or similar:
Supplier: Marshield, Full Wrap Apron, #MS-SP1
Supplier: Universal Medical Inc, Diaper 14" x 20", #800
Wear a PEN dosimeter and/or film badge.
If you are required in the exam room during a procedure, stay as far from the
scanner as possible or behind a mobile protective wall.
The physician is responsible for protecting the patient from unnecessary radiation.
System Safety Devices:
Emergency Stop: In the event of an emergency (any moving component collides with any
parts of the equipment or items in the environment, or that could cause physical injury to the
Patient), the Operator or Patient should utilize one of the 2 designated Emergency Stop buttons
to turn off the power to the X-ray and all moving parts in order for the Patient to be safely
removed from the machine. There is an Operator E-stop button on the Operator Control Box
and there is a Patient E-Stop button on the machine by the seat. The Emergency Stop (s) when
activated will remove ALL power from the machine. If the machine gates are closed, they will
have to be opened manually and any obstructions to the patient exit manually removed.
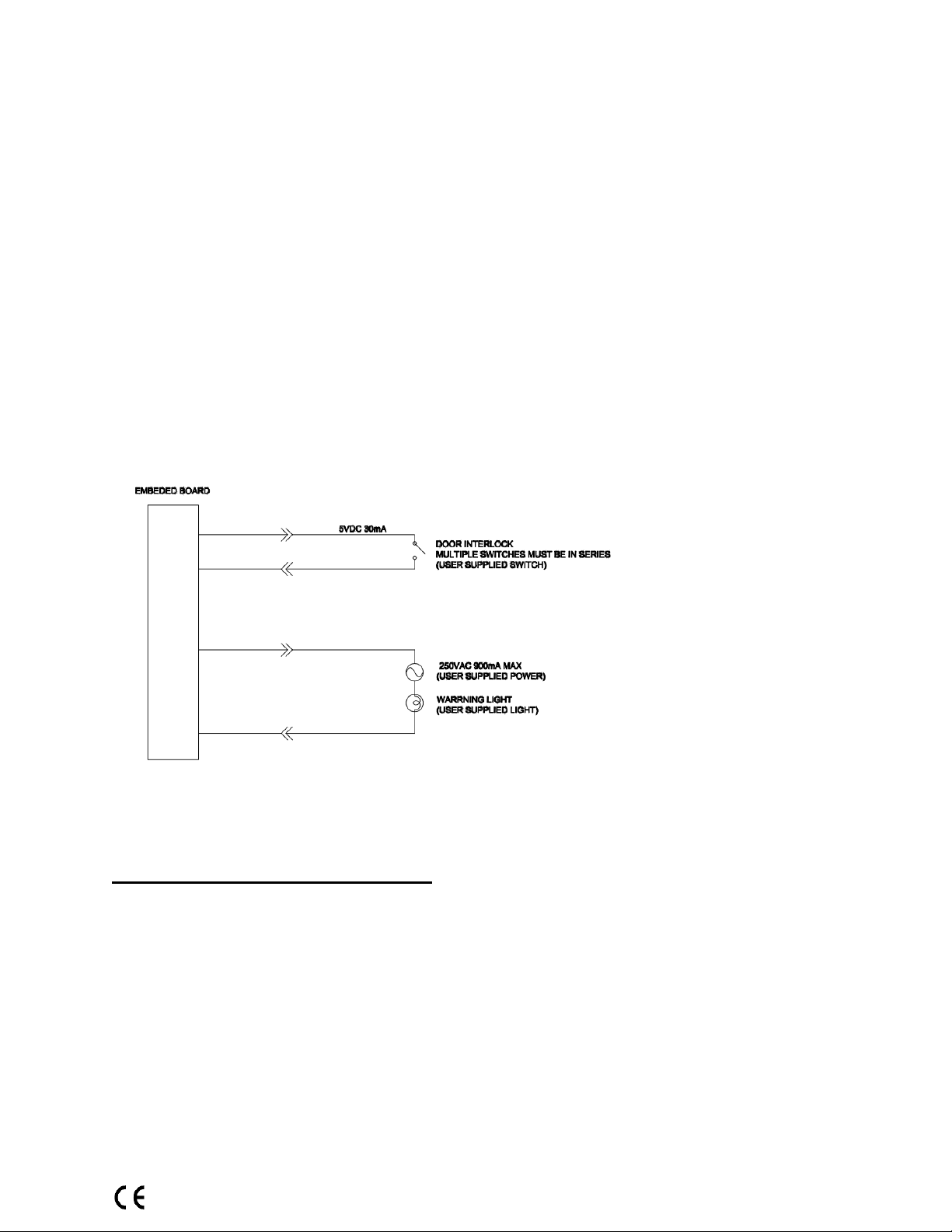
Warning System: The System is equipped with provisions for warning lights and/or audible
alarms when X-ray power is energized. An externally powered Warning System can be