INSTRUCTIONS FOR USE
IFU 03-0235 REV J©2023 Kyra Medical, Inc. 3
1 Instructions for Use:
1.1 Indication for Use:
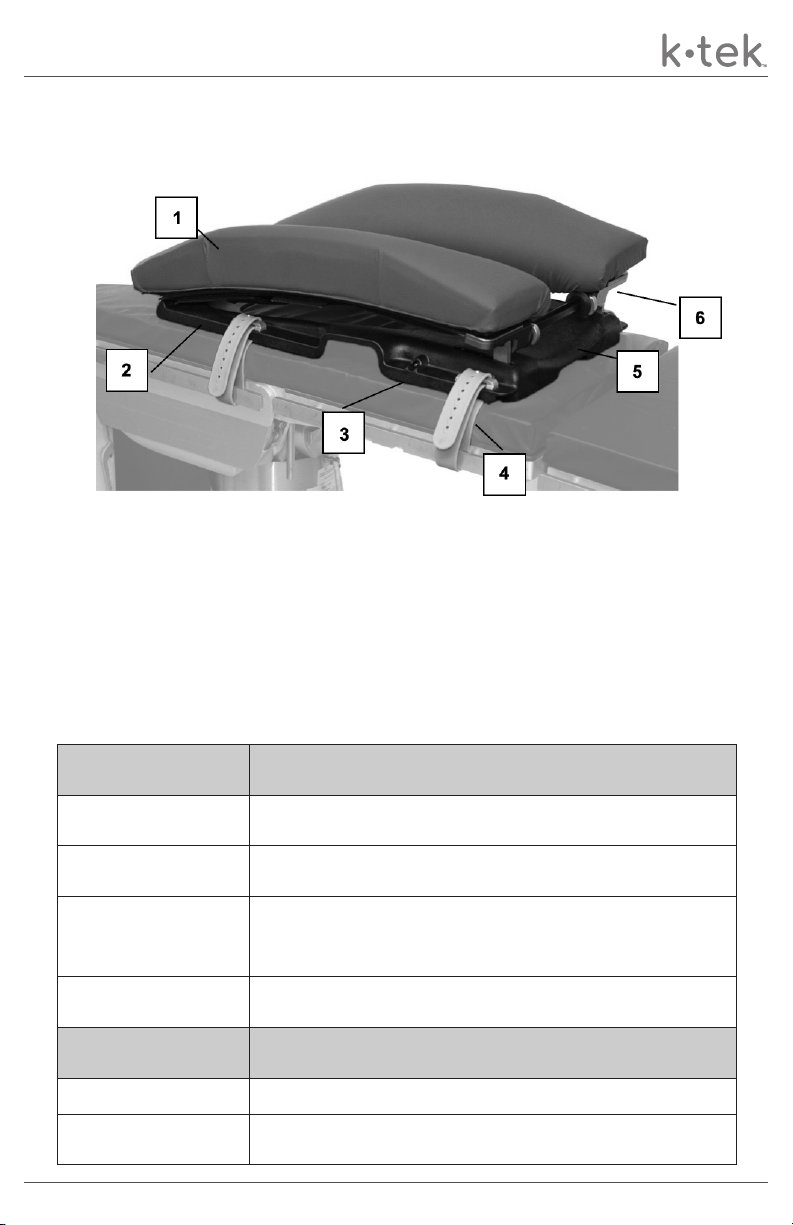
The KTEK Curve spine frame provides maximum lordosis in a variety of surgical spine
procedures including, but not limited to Laminectomy, Decompression, Disc Surgery and
Microdiscectomy surgery. These devices are capable of being used with a broad patient
population as deemed appropriate by the caregiver or institution.
1.2 Intended Use:
The KTEK Curve spine frame is designed to position and support the patient’s spine in a
variety of surgical procedures including, but not limited to Laminectomy, Decompression,
Disc Surgery and Microdiscectomy surgery. These devices are intended to be used by
healthcare professionals within the Operating Room setting.
1.3 Intended User and Patient Population:
Intended User: Surgeons, Nurses, Doctors, Physicians and/or Healthcare profession-
als involved in the intended procedure utilizing the device. Not intended for Lay persons.
Intended Populations: This device is intended to be used with patients that do not
exceed the weight in the safe working load eld specied in the product specication
section 3.2.
1.4 Residual Risk:
This product complies with relevant performance, safety standards. However, device
harm from misuse, device damage, function or mechanical hazards cannot be complete-
ly excluded. User is responsible to ensure device is securely attached and will operate
in a safe manner.
2 Safety Considerations:
2.1 Safety hazard symbol notice:
DO NOT USE IF PRODUCT SHOWS VISIBLE DAMAGE.
2.2 Equipment Misuse:
Do not use the product if package is damaged or unintentionally opened before use. All
modications, upgrades, or repairs must be performed by an authorized specialist.
2.3 Safe Disposal:
Customers should adhere to all federal, state, regional, and/or local laws and regulations
as it pertains to the safe disposal of medical devices and accessories.
If in doubt, the user of the device shall rst contact Kyra Medical Technical Support for
guidance on safe disposal protocols.