5
CONTENTS
01 GENERAL INFORMATION 08
1.1. DEAR CUSTOMER 08
1.2. INDICATION FOR USE 08
1.3. CONTRAINDICATION 08
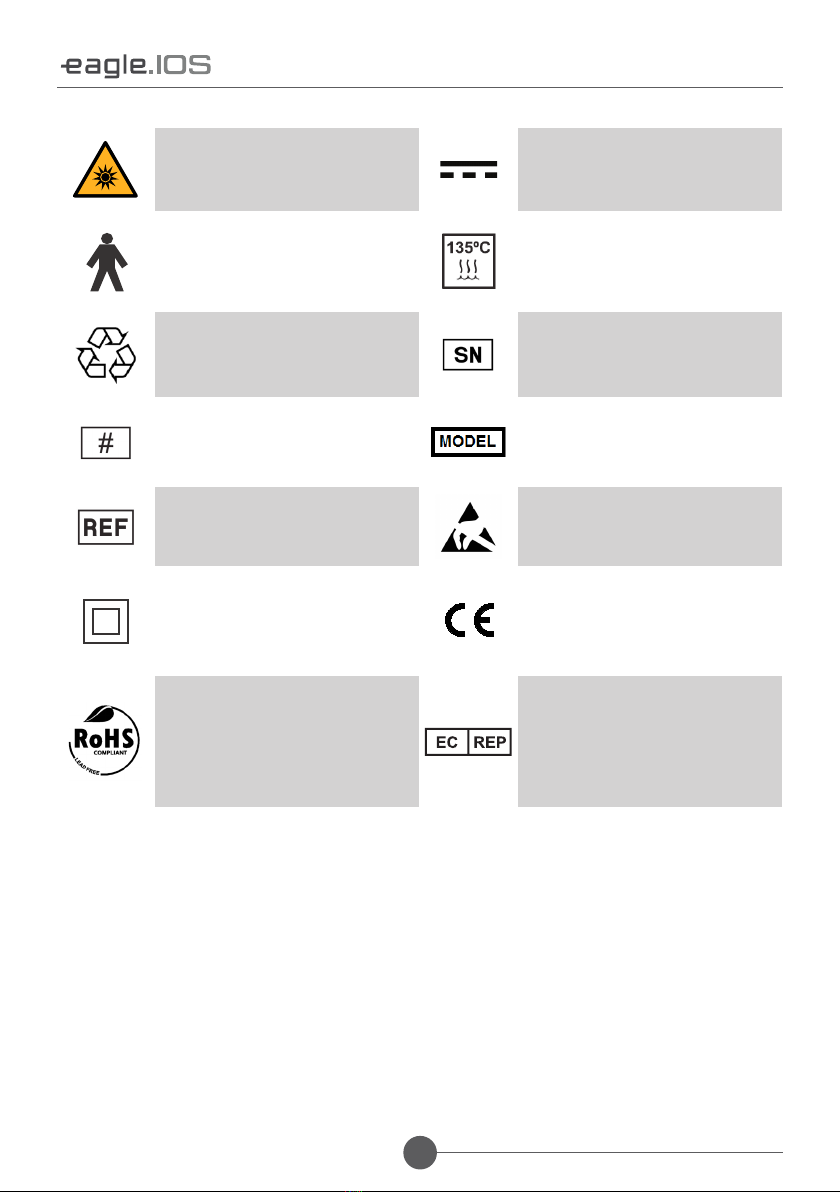
1.4. SYMBOLOGY 09
02 WARNINGS, CAUTIONS AND RECOMMENDATIONS 12
03 SYSTEM GENERAL DESCRIPTION 18
3.1. SYSTEM DESCRIPTION 18
3.2. PRINCIPLES OF OPERATION 18
3.2.1. User Prole 18
3.3. MAIN PRODUCT COMPONENTS 19
3.3.1. Parts that come with the product 19
04 INSTALLATION 21
4.1. EAGLE IOS CONFIGURATION AND CONNECTION 21
4.2. SETTINGS 24
05 OPERATION 31
5.1. INTRODUCTION 31
5.1.1. Communication with the laboratory 31
5.1.2. Case management software overview 33
5.2. WORKFLOW INDICATIONS 36
5.2.1. Create new case 37
5.3. IMPORTANT TIPS BEFORE SCANNING 39
5.4. OPERATE THE SCANNER 39
5.4.1. Scanning Strategy 39
5.4.2. Scanning 40
5.4.3. Mandibular and maxillary scanning 41
5.4.4. Bite alignment 42
5.4.5. Tip 44
5.5. SCANNING TOOLS 45
06 CLEANING, DISINFECTION AND STERILIZATION 47
6.1. INTRODUCTION 47
6.2. CLEANING THE HANDPIECE 47
6.3. CLEANING AND STERILIZATION OF THE TIP 48
07 PROBLEM DIAGNOSIS, INSPECTION AND MAINTENANCE 51
7.1. TROUBLESHOOTING 51
7.2. PERIODIC INSPECTION 51
7.3. PREVENTIVE MAINTENANCE 52
7.4. CORRECTIVE MAINTENANCE 52
08 STANDARDS AND REGULATIONS 55
09 TECHNICAL SPECIFICATIONS 57
9.1. EQUIPMENT CLASSIFICATION 57
9.2. DEVICE INFORMATION 57
9.3. COMPUTER REQUIREMENTS 59