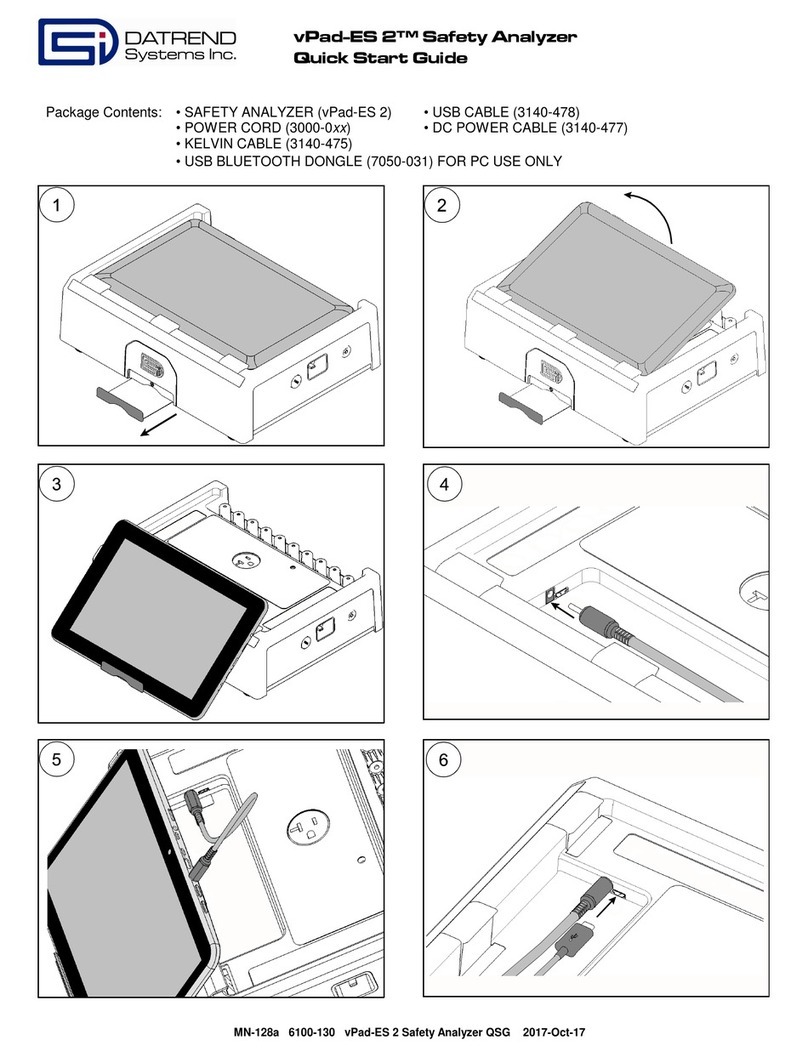
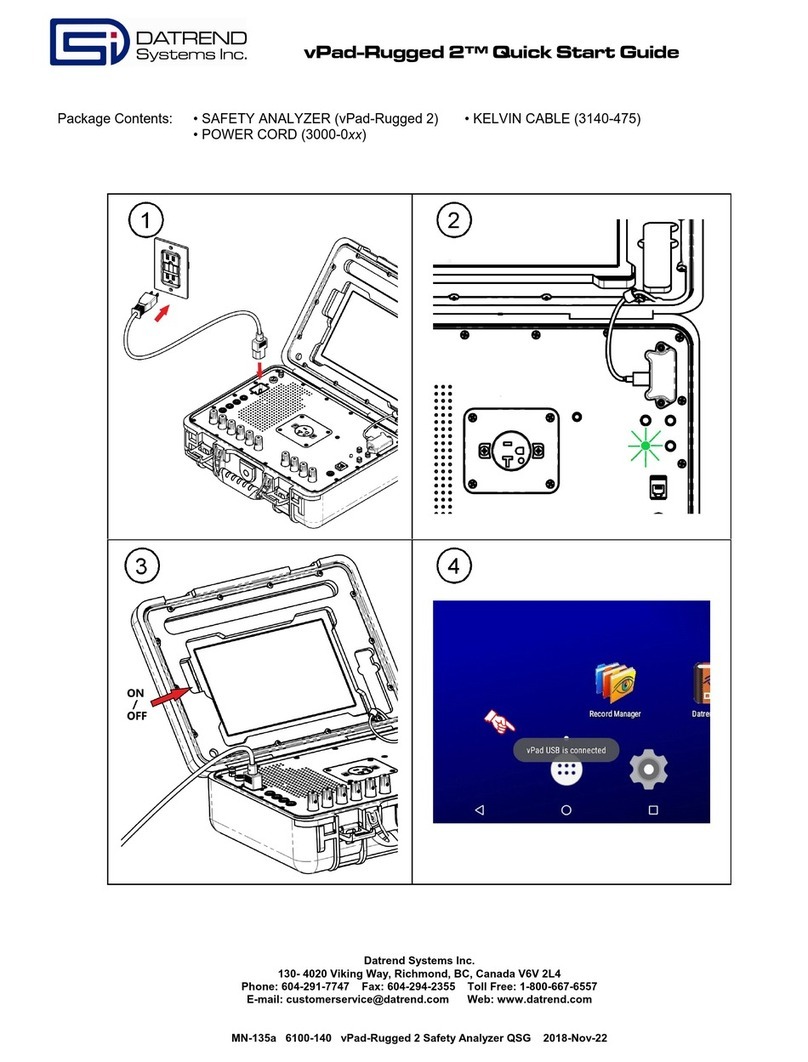
Warranty
Warranty and Product Support
Datrend Systems Inc. ("DSI") warrants each Module of vPad-IV (the "Datrend product") to be free from defects in
materials and workmanship under normal use and service for one (1) year from the date of original purchase. This
warranty will be automatically extended to two (2) years from the date of original purchase, provided that calibration is
performed on an annual basis by a Datrend Authorized Service Center*. During the warranty period DSI will, at our
option, either repair or replace defects in materials and workmanship at no charge; provided the Datrend product is
returned (shipping, duty, brokerage and taxes prepaid) to DSI. Any and all transportation charges incurred are the
responsibility of the purchaser and are not included within this warranty. This warranty extends only to the original
purchaseranddoesnotcoverdamagefromabuse,neglect,accidentormisuseorastheresultofserviceormodification
byotherthanDSI. INNOEVENT SHALLDATREND SYSTEMSINC.BELIABLEFORCONSEQUENTIALDAMAGES.
This warranty is subject to the following limitations:
!Tablet PC: per tablet manufacturer's original warranty
!Standard Accessories: 90 day limited warranty
!Damage to Module caused by use of incompatible test fluids or cleaning agents is not covered under the warranty
!Water/fluid damage to tablet PC is not covered under warranty
!Re-calibration of the instrument, which has a recommended annual calibration frequency, is not covered under the
warranty.
No warranty shall apply when damage is caused by any of the following:
!Power failure, surges, or spikes,
!Damage in transit or when moving the instrument,
!Improper power supply such as low voltage, incorrect voltage, defective wiring or inadequate fuses,
!Accident, alteration, abuse or misuse of the instrument,
!Fire, water damage, theft, war, riot, hostility, acts of God, such as hurricanes, floods, etc.
Onlyserialized products (those items bearing a distinct serial number tag) and their accessory items are covered under
this warranty. PHYSICAL DAMAGE CAUSED BY MISUSE OR PHYSICAL ABUSE IS NOT COVERED UNDER THE
WARRANTY. Items such as cables and non-serialized modules are not covered under this warranty.
This warrantygives you specific legal rights and you may have other rights, which vary from province to province, state
to state, or country to country. This warranty is limited to repairing the instrument to DSI's specifications.
When you return an instrument to DSI for service, repair or calibration, we recommend shipment using the original
shipping foam and container. If the original packing materials are not available, we recommend the following guide for
repackaging:
!Use a double-walled carton of sufficient strength for the weight being shipped.
!Useheavypaperorcardboardtoprotectallinstrumentsurfaces.Usenon-abrasivematerialaroundallprojectingparts.
!Use at least four inches of tightly packed, industrial-approved, shock-absorbent material all around the instrument.
DSI will not be responsible for lost shipments or instruments received in damaged condition due to improper packaging
or handling. All warranty claim shipments must be made on a prepaid basis (freight, duty, brokerage, and taxes). No
returns will be accepted without a Return Materials Authorization ("RMA”) number. Please contact Datrend (refer to
Chapter 5 of this manual) to obtain an RMA number and receive help with shipping/customs documentation.
* Subject to some exclusions, based on sales territory. Contact Datrend for details.
Page ii