Devon extriCARE 2400 User manual

www.devonmedicalproducts.com
1.866.446.0092
ENGLISH
Negative Pressure Wound
Therapy System
extriCARE
®
2400
Operating Manual
IFU30.0003 Rev H 20150403
1. Introduction
The extriCARE®2400 Negative Pressure Wound Therapy Pump System is a portable,
battery-powered pump intended to generate negative pressure or suction to remove
wound exudates, infectious material, and tissue debris from the wound bed which
may promote wound healing. The extriCARE®2400 System consists of one AC power
cable and one 100CC canister. extriCARE®wound dressings, additional canisters,
carrying cases, and other accessories are sold separately. The extriCARE®Negative
Pressure Wound Therapy Pump, when used with the proprietary extriCARE®wound
dressing, creates a negative pressure environment.
The extriCARE®2400 Negative Pressure Wound Therapy pump and extriCARE®wound
dressing are able to produce a negative pressure environment in either intermittent
or continuous mode. This allows the user to program the specific pressure ranging
from 40mmHg to 140mmHg. In continuous mode, the pressure is applied to the
wound as long as the pump is powered on. In intermittent mode, the pump will
alternate between applying pressure for 5 continuous minutes and releasing
pressure for 2 minutes.
Clinically suggested wound types that can be treated using Negative Pressure Wound
Therapy technology are:
• Chronic wounds • Acute wounds
• Traumatic wounds • Subacute and dehisced wounds
• Partial-thickness burns • Ulcers (such as diabetic or pressure)
• Flaps and grafts
The extriCARE®device is meant for continuous use (at least 22 of 24 hours per day).
1
2
0123

2. Symbol List
2
Warning/Caution: See instructions for use
Single Use Only
Date Of Manufacture
Type B. Applied Part. Internally powered electrical device
Keep Dry
Serial Number
Prescription Use Only
Power Switch
Manufacture Lot Number
Biohazard
2
SN
LOT
Class II Equipment
Waste Electrical Goods Recycled

3
4
2. Symbol List (continued)
Authorized Representative in the European Community
Conforms with the Medical Device Directive (93/42/EEC) and
has been subject to the conformity procedures laid down in
the council directive
ETL Listed, Conforms to UL Std. 60601-1
Manufacturer
Catalog / Model Number
Sterilized Using Ethylene Oxide
Use By
0123

3. Device Specifications
DIMENSIONS: Length: 3.35” (8.5cm)
Height: 5.67” (14.4cm)
Width: 1.46” (3.7cm)
WEIGHT: 8.6 oz.
MOBILITY: Portable. Carrying case available.
BATTERY TYPE: Lithium (rechargeable), 3.7V
AC/DC ADAPTER: Input: 100-240 V, 50/60 Hz, 0.4A;
Output: 5V, 1.5A
VACUUM MODES: Continuous or Intermittent
OPERATING CONDITIONS: Temperature: +5°C to 40°C (41°F to 104°F)
Humidity: 15% to 85% non-condensing
PRESSURE OPTIONS: 40mmHg - 140mmHg
FUSE: 1.5A
CHARGING TIME: < 3.5 hours
BAROMETRIC PRESSURE: 800hPa – 1060hPa
STORAGE TEMPERATURE: -10°C to +45°C (14°F to 113°F)
ALTITUDE RANGE: < 2000 m
INGRESS PROTECTION: IPX0
PROTECTION AGAINST
ELECTRICAL SHOCK: CLASS II
PATIENT PROTECTION: Type B
4

5
6
4. Accessories
1. AC/DC Adapter: Input: 100 – 240V, 50/60Hz, 0.4A. Output: 5.0V, 1.5A.
2. Tubing set: 1.55m tubing with a luer-lock connector on one end
preattached. A clamp is also attached to the tubing.
3. Canister: Available in 100 and 400cc configurations.
4. Dressings: Please reference extriCARE®2400 Negative Pressure Wound Therapy
(NPWT) System Dressings (LB30.0004) for a complete listing of all current
dressing options.
5. Carrying case: Now available for 100cc and 400cc canister.
5. Indications for Use
The extriCARE®2400 Negative Pressure Wound Therapy Pump System is indicated for
wound management via the application of negative pressure to the wound by
removal of wound exudate, infectious materials, and tissue debris from the
wound bed. The extriCARE®2400 Negative Pressure Wound Therapy Pump System is
indicated for the following wound types: chronic, acute, traumatic, subacute and
dehisced wounds, partial-thickness burns, ulcers (such as diabetic or pressure),
flaps and grafts.
6. Contraindications for Use
The extriCARE®2400 System should NOT be used in the following conditions:
• Exposed vessels, organs, or nerves.
• Anastomotic sites.
• Exposed arteries or veins in a wound. All exposed vessels and organs in
and around the wound must be completely covered prior to initiation of
NPWT. Note: A thick layer of natural tissue is preferred. Several layers of
fine meshed non-adherent material or bio-engineered tissue may be an
alternative. Ensure that protective materials will maintain their position
throughout therapy.

6. Contraindications for Use (continued)
•Fistulas, unexplored or non-enteric.
• Untreated osteomyelitis.
• Malignancy in the wound.
• Excess amount of necrotic tissue with eschar.
•Wounds which are too large or too deep to be accommodated by the dressing.
•Inability to be followed by a medical professional or to keep scheduled
appointments.
• Allergy to urethane dressings and adhesives.
• Use of topical products which must be applied more frequently than the
dressing change schedule allows.
7. Warnings
•Review this manual prior to using the extriCARE®2400 Negative Pressure Wound
Therapy Pump System. If clarification is needed, contact technical personnel or
Devon Medical Products at 1-866-446-0092 prior to use. Additional questions can
be immediately addressed as well.
•Do not use the extriCARE®2400 Negative Pressure Wound Therapy Pump around
explosive or flammable material. Do not use the pump in an MRI environment
or hyperbaric chamber. Disconnect prior to defibrillation.
• This device should be used only under the direction of a trained professional,
such as a doctor or nurse.
•The 400cc canister should only be used in a facility where drainage can be closely
monitored due to the increased risk of injury to the patient due to bleeding when
using the 400cc canister. Precautionary measures should be taken for patients
who have an increased risk of bleeding (Please see Section 8.1 #1) when using
the 400cc canister.
• Negative Pressure Wound Therapy has not been cleared for use on children.
• Use a properly rated charger to charge the lithium battery. Incorrect
voltage and/or current can cause fire.
• Do not place this device at temperatures greater than 170°F for more
than 2 hours, as it may cause a battery fire.
• If battery swells, gets hot, or smokes while charging, disconnect the charger
immediately. This may cause the battery to leak, and the reaction with air may
cause the chemicals to ignite, resulting in fire.
6

7
8
8. Precautions
8.1) Be aware for any of the following conditions:
There are additional conditions to take into account before using Negative
Pressure Wound Therapy, such as:
1. BLEEDING: There is a risk of bleeding/hemorrhaging with negative
pressure wound therapy. If hemostasis cannot be achieved, if the patient
is on anticoagulants or platelet aggregation factors, or if the patient has
friable blood vessels or infected vascular anastomosis, he or she may
have an increased risk of bleeding; accordingly these patients should be
treated in an inpatient care facility per their treating physician.
If active bleeding develops suddenly or in large amounts during
therapy, immediately disconnect the pump, leave the extriCARE®wound
dressings in place, and take measures to stop bleeding. Seek medical
attention immediately.
2. VESSEL AND BONE PROTECTION: Precautionary measures should be
taken if any bones, vessels, ligaments or tendons are exposed.
Additionally, sharp edges (due to bone fragments) require special
attention; these areas should be covered and smoothed wherever
possible. These conditions should be factored into the therapy
prescription as the attending clinician sees fit.
3. ENVIRONMENT:
a. Defibrillation: Remove the extriCARE®dressing if defibrillation is
required in the area of dressing placement. Failure to remove the
extriCARE®wound dressings may inhibit transmission of electrical
energy and/or patient resuscitation.
b. Magnetic Resonance Imaging (MRI): The extriCARE® device is unsafe in
the MR environment. Do not take the extriCARE®device into the MR
environment. extriCARE®dressings however can typically stay on the
patient with minimal risk in an MR environment, assuming that the use
of the extriCARE®Negative Pressure Wound Therapy System is not interrupted
for more than two hours.
c. Hyperbaric Oxygen Therapy (HBO): Do not take the extriCARE®device
into a hyperbaric oxygen chamber. extriCARE®devices are not designed
for this environment, and should be considered a fire hazard in such
an environment. After disconnecting the extriCARE®device, either (i)
replace the extriCARE® dressing with another HBO compatible material
during the hyperbaric treatment, or (ii) cover the unclamped end of the
extriCARE®tubing. For HBO therapy, the extriCARE®tubing must not be
clamped. Never leave an extriCARE®dressing in place without active
extriCARE® Negative Pressure Wound Therapy for more than two hours.

8
8.1) Be aware for any of the following conditions (continued):
4. INFECTION: Infected wounds and osteomyelitis pose significant
risks for Negative Pressure Wound Therapy.If untreated osteomyelitis is
present, therapy should not be initiated. Negative Pressure Wound Therapy
should not be used to treat infections, and all infections should be treated
and addressed prior to using the extriCARE®Negative Pressure Wound Therapy
System.
5. PATIENT SIZE AND WEIGHT: Patient size and weight should be taken
into account when prescribing therapy. In addition, small adults, young
adults or elderly patients should be closely monitored.
6. SPINAL CORD INJURY: If a patient experiences autonomic dysreflexia
(sudden changes in blood pressure or heart rate because of sympathetic
nervous system stimulation) discontinue extriCARE®therapy to minimize
sensory stimulation and give immediate medical assistance.
7. MODE: In unstable anatomical structures, continuous rather than
intermittent therapy is recommended to help minimize movement and
instability. Continuous therapy is also recommended in patients with an
increased bleeding risk, profusely exudating wounds, fresh grafts and/or
flaps, and wounds with acute enteric fistulae.
8. ENTERIC FISTULAS: Wounds with enteric fistulas require special
consideration to be effective in negative pressure wound therapy.
If enteric fistula effluent management or containment is the only goal
of such therapy, extriCARE®is not recommended.
9. CIRCUMFERENTIAL DRESSING: Do not use circumferential dressings.
10. BRADYCARDIA: Avoid placement of the extriCARE®2400 Negative Pressure
Wound Therapy Dressings next to the vagus nerve to minimize the risk of
bradycardia.
NOTE: If any of this information is not understood, contact the manufacturer before using the
device.

9
10
8.2) Prior to Therapy
• Patient should be assessed and measures should be taken to optimize
and stabilize their medical condition. Nutrition, medication, blood
glucose, blood pressure, and circulation as well as other medical issues
should be addressed.
• The wound should be recently debrided by whatever measure is
appropriate and the amount of necrotic tissue should be minimized.
• Issues of infection should be addressed.
8.3) Periwound Skin
•Ensure that the skin that will be under the dressing is clean, dry, free of
surfactants and oil. Any hair should be clipped.
• The periwound area should be cleaned and allowed to air dry. The use
of a skin preparation wipe is also recommended.
• A thin film dressing or hydrocolloid may be used as additional
protection.
• Monitor skin for signs of irritation or breakdown. Treatment may be
discontinued if this occurs and cannot be managed.
8.4) Dressing Management
The extriCARE®dressing is a one piece all inclusive dressing and should
be removed in one piece. In the event that the extriCARE®wound dressings
comes apart, all extriCARE®wound dressings materials must be removed from
the wound prior to further treatment.
Clean and debride the wound as necessary. Any bleeding should be
controlled. Follow facility protocol for wound prep and infection control.
The type of extriCARE®wound dressings chosen for use is dependent on
the wound type, size, and location. extriCARE®wound dressings size and
type is labeled on each package.
•Care should be taken to avoid stretching of the dressing.
•Avoid pleating the extriCARE®wound dressings. Additional tape and
urethane may be applied to secure the extriCARE®dressing in place.
• Do not use as a circumferential dressing.
•Additional wrap dressing may be applied over the extriCARE®wound
dressings to further secure the extriCARE®wound dressings and provide
additional support.
•If used on anatomically challenging areas or where adhesion is a
problem, a thin layer of ostomy paste may be applied.
• Refer to instructions for specific information regarding each extriCARE®
wound dressings.

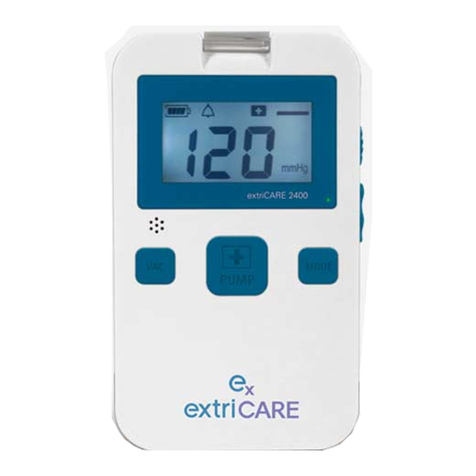
9. Features
9.1) Defined Features
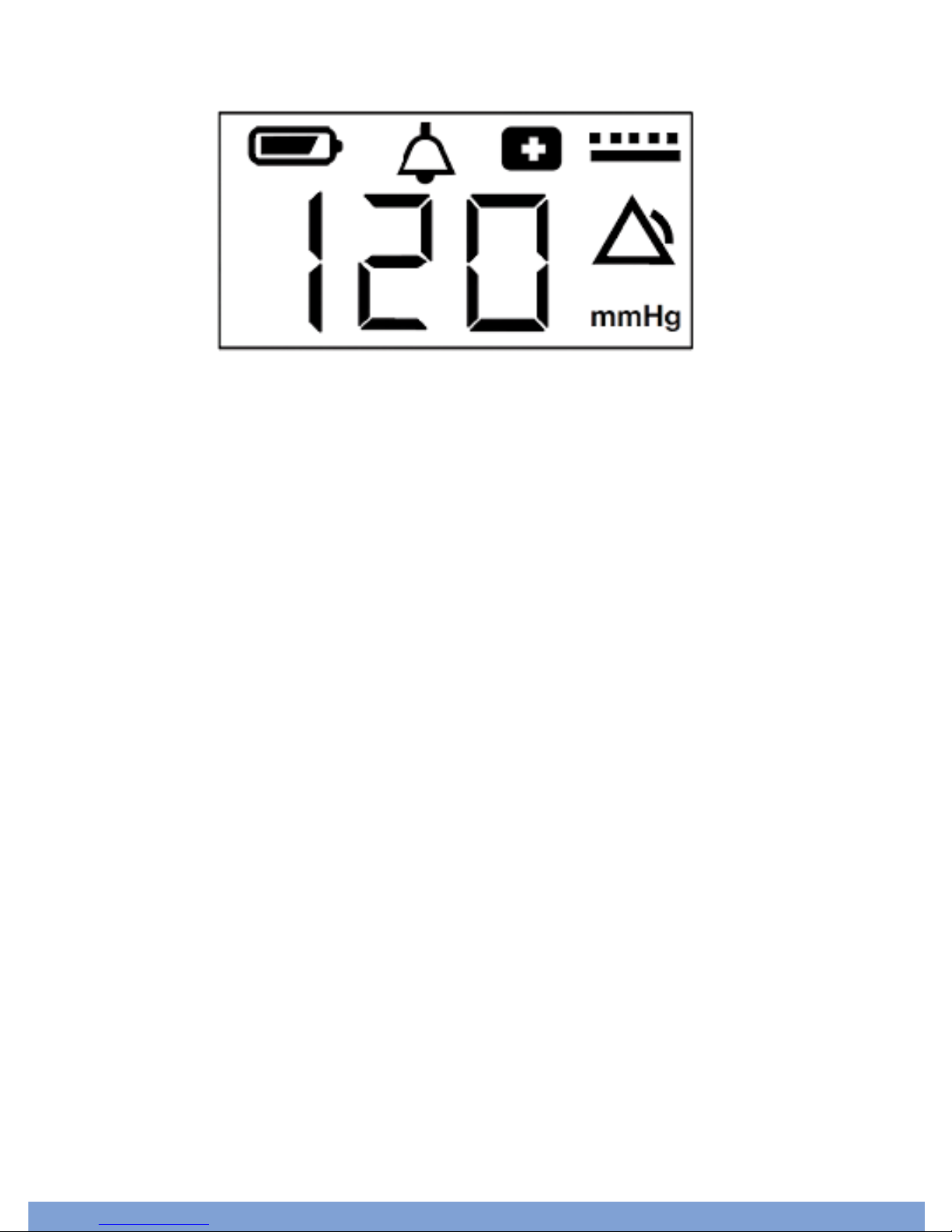
1. LCD SCREEN: Indicates the pump operating pressure and displays
symbols (also features a blue backlight).
2. CONNECTION TUBING: Tubing which connects canister to drainage
tubing.
3. BATTERY POWER: Indicates how much battery power is left. Icon has 1-4
bars representing 25%, 50%, 75%, and 100% battery power.
4. CANISTER CLIP: Clip that connects the canister to the NPWT device.
5. MODE SYMBOL: Indicates pump operating mode (continuous or
intermittent).
6. MODE BUTTON: Allows user to set the pump to either continuous or
intermittent mode.
7. POWER PLUG: Enables user to charge device.
8. PUMP BUTTON: Used to turn pump on or off. Also can be used to
exit a setting.
9. SET BUTTON: Used to program desired pressure.
10. POWER SWITCH: Used to turn the system power on and off.
3.) Battery Power
1.) LCD Screen
9.) SET Button 6.) Mode Button
8.) Pump Button
7.) Power Plug
4.) Canister Clip
5.) Mode Symbol
10.) Power Switch
2.) Connection Tubing
10
11
12
9.2) Alarm Features/Troubleshooting
In order to assure proper patient compliance, the extriCARE®2400 system is
equipped with both audio and visual alarms for all the errors listed in the chart
below.
To disengage the alarms:
1. Audio alarms can be muted by pressing any button on the device front plate.
2. Visual alarms, which include the LED and screen symbols, need to be
disengaged by pressing the “PUMP” button. If the screen is locked, it needs to
be unlocked first before the visual alarm can be disengaged. For instructions
on how to unlock the screen please refer to section 10.2.5.
Canister Full
Error
Tilt Error
Low Battery
Error
High Voltage
Error
E01
E03
E04
E05
The canister is
equipped with full
sensors that will be
triggered either when
a canister is full of
exudates or a false
fullness caused by
incorrect use of the
system
The extriCARE®
System is tilted at an
angle greater than 95
degrees with respect
to the upright position
for more than 10
seconds
Less than 25% power
remaining indicat-
ing that extriCARE®
System will power off
soon
The extriCARE® Sys-
tem being used with
an adapter that is not
recommended;
There is a risk of volt-
age incompatibility if
the input voltage is
greater than 6V
3 beeps
every 20
seconds
3 beeps
every 20
seconds
3 beeps
every 20
seconds
3 beeps
every 20
seconds
Alarm symbol and
“E01” flashing on
LCD screen
Yellow LED
flashing every 2
seconds
Alarm symbol and
“E03” flashing on
LCD screen.
Yellow LED
flashing every 2
seconds
Battery, alarm
symbol and “E04”
flashing on LCD
screen
Yellow LED
flashing every 2
seconds
Alarm symbol and
“E05” flashing on
LCD screen
Yellow LED
flashing every 2
seconds
Pump will
shut off
immedi-
ately
Pump re-
mains on
Pump
remains in
function
until the
battery
depletes
System is
shut off
Install a
new
canister
Place the
device
back to an
upright
position
Plug the
extriCARE®
System in,
allowing it
to function
and charge
simultane-
ously
Unplug the
adapter and
use the rec-
ommended
adapter
Error
Code Error Type Cause Audio Alarm
Features
Visual Alarm
Features
System
Status
Suggested
Mitigation

12
9.2) Alarm Features/Troubleshooting (continued)
Canister
Installation
Error
Blockage
a) Major
Leak Error
b) Minor
Leak Error
E06
E07
E02
E08
The canister is not de-
tected or is installed
incorrectly
Tubing or dressing
clog or blockage
Pump unable to reach
50% of the preset
pressure after 1 min-
ute of pumping effort
Pump unable to reach
80%
of the preset pressure
after 1 minute of
pumping effort
3 beeps
every 20
seconds
1 beep
every 20
seconds
3 beeps
every 20
seconds
1 beep
every 20
seconds
Alarm symbol and
“E06” flashing on
LCD screen
Yellow LED
flashing every 2
seconds
Alarm sym-
bol and “E07”
flashing on LCD
screen. Yellow
LED always on
Alarm symbol and
“E02” flashing on
LCD screen
Yellow LED
flashing every 2
seconds
Alarm symbol and
“E08” flashing on
LCD screen, yel-
low LED remains
illuminated
Pump will
not run
Pump re-
mains on
System
will shut
down after
5 minutes
System
will shut
down after
20 minutes
Properly
install the
canister in
place
Replace the
dressing
and tubing
set with a
new set
Inspect for
possible
air leaks
between:
-the
wound and
extriCARE®
dressing
-the
extriCARE®
dressing
and canister
-the can-
ister and
pump
- if neces-
sary, power
off and
back on
to restart
Error
Code Error Type Cause Audio Alarm
Features
Visual Alarm
Features
System
Status
Suggested
Mitigation
Air Leakage Error There are many potential sources of leaks (incomplete seal between
extriCARE®dressing and skin, improper connection between tubing,
canister leakage, etc.) The alarms have been divided into two categories:

13
14
10. Instructions for Use
10.1) Dressing and Canister Application
The clinician may loosely place extra non occlusive dressing material into areas of
undermining and tunneling. The decision type of non occlusive material used is based on
clinician preference. Document the amount of additional packing material used.
extriCARE®wound dressings should be changed as needed.
•The initial extriCARE®wound dressings should be changed in 24 - 48
hours or when leaking, whichever comes first. extriCARE®wound
dressings should not be left in place longer than 72 hours.
•If the extriCARE®wound dressings sticks to the wound, moisten with
saline or water during removal. Adhesive remover may be used.
•Dispose of soiled extriCARE®wound dressings according to facility
protocol.
Avoid outside sources wetting the extriCARE®wound dressings. The
extriCARE®wound dressings should be protected from moisture during bathing
or changed prior to reconnecting to the pump. Do not use the extriCARE®
2400 Negative Pressure Wound Therapy Pump while showering or bathing. Always
disconnect and remove pump from areas of moisture (bathing area or tub).
Clamp the tubing when pump is disconnected.
To remove a canister, pull up on the canister clip on the top of the device and
pull the canister away. To reinstall a canister, line up the notch on the bottom
of the canister with the hole for it on the extriCARE®pump, and then press the
canister clip into place. The clip should click into place and the canister should
feel snug.

14
10.1) Dressing and Canister Application (continued)
When using on a venous or other leg ulcer:
•Edema control must continue during wound treatment.
• Consider lower pressures when applied over fragile skin.
When applying the extriCARE®wound dressings over toes:
• A thin layer of petroleum jelly or other oil based ointment should
be applied to nails.
• Additionally, antifungal medication and a small amount of soft
dressing material may be applied between each toe.
When used on the foot, aggressive measures should be taken to protect
the foot and divert unnecessary pressure.
If the extriCARE®wound dressings is applied over a new graft or
bioengineered tissue:
•It is recommended that a non-adherent open weave or fenestrated
silicone contact layer be applied atop the wound between the graft
and the NPWT dressing.
•Heavy petrolatum or similar products cannot be used as negative
pressure will not have an impact on the wound surface.
• Additional care should be used during dressing change to prevent
dislodging graft.
15
16
10.2) Operating the Device
LCD Display
1. POWER ON/OFF: To Power on the device, push the POWER SWITCH on
the right side of the device downward. The device should then turn on.
Push the POWER SWITCH upward to turn device off.
2. CONTROL PRESSURE: Holding down the SET key for two seconds will
initiate the procedure for setting the pressure. The screen will display a
flashing pressure reading at this time. Press SET once to increase
pressure by 20mmHg. In order to obtain a lower pressure, scroll through
by pressing SET button. The pressure will increase until 140mmHg, and
then will start at 40mmHg again. When desired pressure is reached, press
the PUMP button to confirm and exit pressure settings.
3. SET MODE: Hold down MODE button for two seconds to select the
mode (continuous or intermittent). A dotted line at the top right of the
LCD Screen indicates intermittent treatment while a straight line indicates
a continuous treatment. To change current mode, press the MODE button.
To exit Mode Settings, press the PUMP button.
4. START/STOP TREATMENT: To start treatment, hold the PUMP button for
two seconds. Do the same to stop treatment.
5. LOCK: The locking feature prevents the settings from being changed.
If no buttons on the device are pressed for more than 60 seconds, the lock
will automatically turn on. If the Lock is on and buttons are accidentally
pressed, nothing will be changed. Press the SET and MODE button
simultaneously for 2 seconds to turn the lock on manually. Repeat for 3
seconds in order to unlock. The backlight should turn off when the device is
locked and turn back on when it is unlocked.

16
10.3) Disposal
The extriCARE®Negative Pressure Wound Therapy Pump is powered
electromechanically by a battery that should be recycled according to the
local regulations governing such products and Waste Electrical and Electronic
Equipment (WEEE) Directive.
The extriCARE®wound dressings, tubing, and canister can be disposed of
according to policy for wound care dressings after use.
10.4) Maintenance and Replacement Parts
The extriCARE®device contains no user serviceable parts inside: Opening or
tampering with this device will void the warranty. In the event the extriCARE®
device requires repairs, it should be returned to the medical equipment
company or to Devon Medical Products directly
Power adapter: The extriCARE®device should only be recharged using the AC/
DC adapter provided or an equivalent IEC 60601-1 compliant adapter with a
+5V 1.5A output.
Battery: Do not attempt to open, disassemble, or service the battery pack. Do
not crush, puncture, short external contacts, or dispose of in fire or water. Use
only a Devon Medical Products approved battery. If the device will not be in
use for an extended period of time, the battery should be maintained by
recharging regularly. Battery should be stored in a safe and dry place.
10.5) Cleaning
To clean the extriCARE®Device, follow normal protocol for cleaning medical
devices. If additional information is required, please contact your local
representative or the manufacturer.

17
18
10.6) Pump Operation Tips
1. Fluid Stagnation in Tubing (aka, “Vapor Lock”): This rare condition may
occur when an exceptionally tight seal of the wound dressing facilitates a
situation which interferes with fluid flow, preventing the proper removal
of exudates from the wound site. This phenomenon is caused by a loss of
differential pressure between the wound site and pump, which drives the
motion of fluid removal. Another contributing cause of this problem is if the
pump is placed too high above the wound site.
The potential adverse consequences of fluid stagnation are: 1) pooling of
exudates at the wound site, and 2) the potential of exudate backflow from the
tubing into the wound. Should fluid stagnation occur, the step by step guide
below should be followed to resolve this issue:
• To avoid the gravitational burden that could amplify or cause fluid
stagnation, the extriCARE®2400 device should be placed no higher than 1
meter above the dressing/wound location.
• To avoid differential pressure related stagnation, a ventilation patch should
be used over the existing NPWT dressing to supply the pressure difference
required to effectively remove the exudates from the wound site.
No or extremely low flow
of exudates in tubing and
tubing filling with fluid.
Wound Site:
Is Fluid Pooling
at Dressing?
Fluid Pooling
Fluid NOT Pooling
Low Exudate Wound:
Place ventilation
patch on the NPWT
clear film dressing
(please refer to patch
placement
instructions)
Vapor Lock:
Blockage Alarm
1. Place ventilation patch on the NPWT clear
film dressing (please refer to patch placement
instructions)
2. If step 1 does not resolve Vapor Lock, replace
dressing and tubing (ensure that the dressing
application instructions are properly followed)
Excessive Dressing Leakage:
• Leakage Alarm
• Consider Larger Pump
(3600)
• Replace or Modify Dressing
• Consider Use of silicone or
ostomy strips prior to film
use, add additional film and
wraps, cover connector
with additional film to
improve security
• Use finger pressure to
determine area of leakage
and fix accordingly
Pump Running
Pump NOT Running
Pump Running or
Not Running to
maintain Pressure?

18
11. Warranty Information:
LIMITED WARRANTY
Devon Medical Products warrants its extriCARE®Negative Pressure Wound Therapy
Pump (“Device”) to be free from defects in workmanship and materials for
a period of one (1) year from the date the Device is delivered to the original
purchaser (“Warranty Period”). This Limited Warranty is extended only to
the original purchaser and is non-transferable. Devon Medical Products’ sole
obligation under this Limited Warranty shall be, at its sole discretion, to repair
or replace a Device which is defective in either workmanship or material. This is
the sole remedy of the Purchaser. This Limited Warranty excludes the battery,
canister, canister clip, power plug, connection tubing, and dressings. In addition,
this Limited Warranty does not cover any Device which may have been damaged
in transit or has been subject to misuse, neglect, or accident; or has been used in
violation of Devon Medical Products’ instructions, including, without limitation,
the instructions contained in the Operation Manual.
THERE ARE NO OTHER WARRANTIES THAN THOSE EXPRESSLY STATED HEREIN.
TO THE EXTENT PERMITTED BY LAW, DEVON MEDICAL PRODUCTS DOES NOT MAKE ANY
IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE AS
TO ANY PRODUCT OR DEVICE, WHETHER OR NOT THAT PRODUCT OR DEVICE IS COVERED BY
ANY EXPRESS WARRANTY CONTAINED HEREIN.
IN NO EVENT SHALL DEVON MEDICAL PRODUCTS BE LIABLE FOR ANY SPECIAL, INCIDENTAL,
CONSEQUENTIAL, OR INDIRECT DAMAGES (INCLUDING, WITHOUT LIMITATION, DAMAGES
FOR LOSS OF PROFITS, USE OR TIME INCURRED BY PURCHASER OR END USER). IN ADDITION,
DEVON MEDICAL PRODUCTS SHALL NOT BE LIABLE FOR ANY EXEMPLARY OR PUNITIVE
DAMAGES.
12. Contact Information
Manufactured For:
Devon Medical Products
1100 1st Avenue, Suite 202
King of Prussia, PA 19406, USA
www.devonmedicalproducts.com
+1.866.446.0092
Pump Running

19
20
12. Contact Information (continued)
Obelis s.a
Bd. Général Wahis 53
1030 Brussels, BELGIUM
Tel: +32.2. 732.59.54
Appendix 1
Product Classification:
•According to the type of protection against electrical shock, this device is
classified as a Class II Equipment, and Type B Equipment that is powered by an
external electrical power source.
•According to the degree of protection against harmful ingress of water
this system is classified as Ordinary Equipment (IPXO: without protection
against ingress of water)
•CAUTION: This device has been tested and confirmed to comply with the
IEC 60601-1-2:2007 and essential requirements of Medical Device Directive
93/42/EEC. However with the proliferation of radio-frequency transmitting
equipment, and other sources of electrical noise in a healthcare
environment, high levels of interference may induce an abnormal stoppage
or other disruption of this device. This device may also cause adverse effects
in other nearby equipment. It is strongly recommended that this device be
isolated from other electromagnetic equipment when in use.
•This system is classified as Equipment not Suitable for use in the presence
of a Flammable Anesthetic Mixture with Air or Oxygen or Nitrous Oxide.
•According to the mode of operation this system is classified as Equipment
that can be used for Continuous Operation.
•CAUTION: In the USA, Federal Law restricts this device to sale, by or on the
order of a physician.
•Unit is packaged for transportation by common carrier.
Other manuals for extriCARE 2400
1
Table of contents
Other Devon Medical Equipment manuals