Diesse VES MATIC Original User manual

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 1
USER MANUAL
Rev. 1.0 –September, 2022
Automated instrument for ESR determination
with modified Westergren method
Software version 1.xx.xx
For in vitro diagnostic use only

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 2
This page is intentionally left blank.

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 3
VES MATIC Original models:
This manual is applicable to the following VES MATIC Original instrument models:
Catalogue number
Description
10340
VES MATIC Original
VES MATIC Original Accessories:
Catalogue number
Description
10270
TEST DEVICE ORIGINAL 4K (4000 tests)
10430
ESR Control (4x9 mL)
10434
ESR Control (2x9 mL)
10403
Thermal paper
20550510
External barcode reader
Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 4
Manual revisions list:
REV. User manual
Description
1.0 –September, 2022
First issue

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 5
MANUFACTURER
DIESSE DIAGNOSTICA SENESE S.p.A.
Strada dei Laghi 39, 53035 Monteriggioni (SI) Italy
Tel. +39 0577 307109, Fax. + 39 0577 307106
WWW.DIESSE.IT
TECHNICAL SUPPORT
Strada dei Laghi 39, 53035 Monteriggioni (SI) Italy
Tel. +39 0577 307109, Fax. +39 0577 307106
Toll-free number: 800 606932
e-mail: technicalsupport@diesse.it
If any serious incident has occurred in connection with this device in the European Union
market territory, please report it without delay to the manufacturer and competent
authority of your Member State.
No page in this manual may be reproduced in any form or by any means, electronic,
mechanical or otherwise, for any use whatsoever without prior written permission from
DIESSE DIAGNOSTICA SENESE S.p.A.

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 6
Symbols:
Key of graphic symbols
Instrument meeting the requirements of the EU Regulation 2017/746
on in vitro diagnostic medical devices
In vitro diagnostic medical device
Manufacturing date
Serial number
Manufacturer
Instrument that complies with MET standards for the Canadian and
US markets
Key of electric and safety symbols
Protective conductor
WEEE: Waste electrical and electronic equipment –Mandatory
Separate Collection pursuant to Italian Legislative Decree No. 49 of 14
March, 2014, implementing Directive 2012/19/EC.
Attention: please read this manual carefully and comply with the
safety symbols.
Caution: risk of electrical shock
Key of graphic symbols used in this document
WARNING: potential risk of personal injury; all the conditions indicated
in the relevant text must be read and understood before proceeding.
CAUTION: potential risk of damage to the instrument; all the
conditions indicated in the relative text must be read and understood
before proceeding.
N.B. important information.
BIOHAZARD: risk of contamination with potentially infected
substances.
Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 7
LIMITATIONS AND WARNINGS
Before installation and use of the instrument, for proper and safe use, it is advisable to
read carefully the warnings and instructions in this user manual. It is important that this
user manual be kept together with the instrument for future reference.
In the event of a sale or transfer, make sure that this manual accompanies the
instrument to allow new users to be informed about the instrument’s functions and the
related warnings.
It is recommended to allow only qualified and skilled laboratory personnel to use the
instrument.
The safety and performance requirements of the instrument can no longer
be guaranteed when the instrument is powered using a different cable from
the one supplied, compatible with the power supply of the country of
installation.
BIO-CONTAMINATION HAZARDS
Potentially infected material may be handled.
When using an analysis system such as ESR MATIC Original, all precautions
regarding biohazards must be taken. The samples do not require preparation.
The samples must be disposed of in compliance with laboratory guidelines
and local laws.
Observe personal and group safety measures required for the operator and
appropriate for the work environment. Comply with directives on safety and
the applicable laws in force.
In the event of biological material leakage during the operating cycle, clean
the external surfaces of the instrument with personal protective equipment
and follow the decontamination rules.
All supplied materials must be disposed of in compliance with the local laws.

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 8
Contents
1INTRODUCTION..............................................................................................................................................................10
1.1 Intended use..................................................................................................................................................10
1.2 Instrument overview.................................................................................................................................10
1.3 Clinical significance of ESR ................................................................................................................... 12
1.4 Normal ESR values (Westergren citrated) .................................................................................. 13
1.5 Material required for using the instrument ...............................................................................14
1.6 Warnings..........................................................................................................................................................14
1.7 Personal protective equipment (PPE)........................................................................................... 15
1.8 Ordinary maintenance ............................................................................................................................ 15
1.8.1 Cleaning and decontamination................................................................................................ 15
2TECHNICAL DATA..........................................................................................................................................................17
2.1 Technical description ............................................................................................................................... 17
2.2 External connections of the instrument ......................................................................................18
2.3 Software Update..........................................................................................................................................19
2.4 Technical features......................................................................................................................................20
2.5 Instrument Composition........................................................................................................................ 21
2.6 Units of Measurement ............................................................................................................................. 21
2.7 Instrument Label........................................................................................................................................ 22
3INSTALLATION ................................................................................................................................................................23
3.1 Transport and Handling......................................................................................................................... 23
3.2 Packaging Characteristics.................................................................................................................... 23
3.3 Materials Provided..................................................................................................................................... 23
3.4 Unpacking the Instrument..................................................................................................................24
3.5 Operating Environment ........................................................................................................................ 25
3.6 Installation Procedure............................................................................................................................. 26
3.7 Disposal............................................................................................................................................................ 26
3.8 Fuse replacement...................................................................................................................................... 27
3.9 Procedure for replacing the printer paper ................................................................................28

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 9
4USE.........................................................................................................................................................................................30
4.1 Sample preparation..................................................................................................................................30
4.2 Test tube labelling.....................................................................................................................................30
4.3 Home page .................................................................................................................................................... 32
4.4 START - Performing an analysis cycle ...........................................................................................34
4.4.1 Inserting the test tubes.................................................................................................................36
4.4.2 Starting the analysis cycle........................................................................................................... 37
4.4.3 Inserting a new sample (random access)...........................................................................41
4.4.4 Results......................................................................................................................................................43
4.4.5 Guided reading of the results printout ...............................................................................45
4.4.6 Interruption of a work session ..................................................................................................48
5ARCHIVE ............................................................................................................................................................................ 49
5.1 Sample archive functionality..............................................................................................................50
5.1.1 ‘EXPORT’ button................................................................................................................................50
5.1.2 Samples selection............................................................................................................................. 52
6SETTINGS............................................................................................................................................................................56
7QUALITY CONTROL.................................................................................................................................................... 60
7.1 QC registration procedure ...................................................................................................................60
7.2 Performing QC analysis..........................................................................................................................60
7.3 QC archive.......................................................................................................................................................62
8USER MANAGEMENT.................................................................................................................................................67
9TROUBLESHOOTING..................................................................................................................................................70
9.1 Temperature out of range..................................................................................................................... 71
10 CONNECTION TO HOST COMPUTER ..............................................................................................................73
11 PERFORMANCES..........................................................................................................................................................74
11.1 Precision of VES MATIC Original.......................................................................................................74
11.2 Correlation of VES MATIC Original and Westergren reference method.................78
12 BIBLIOGRAPHY..............................................................................................................................................................85

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 10
1INTRODUCTION
1.1 Intended use
The VES MATIC Original is an automated instrument for the quantitative Erythrocyte
Sedimentation Rate (ESR) determination with the modified Westergren method using
venous whole blood anticoagulated in citrate.
ESR is a non-specific parameter of inflammatory status used as an aid for monitoring the
physiological or pathological state of the patient.
The instrument is to be used only by professional laboratory users.
1.2 Instrument overview
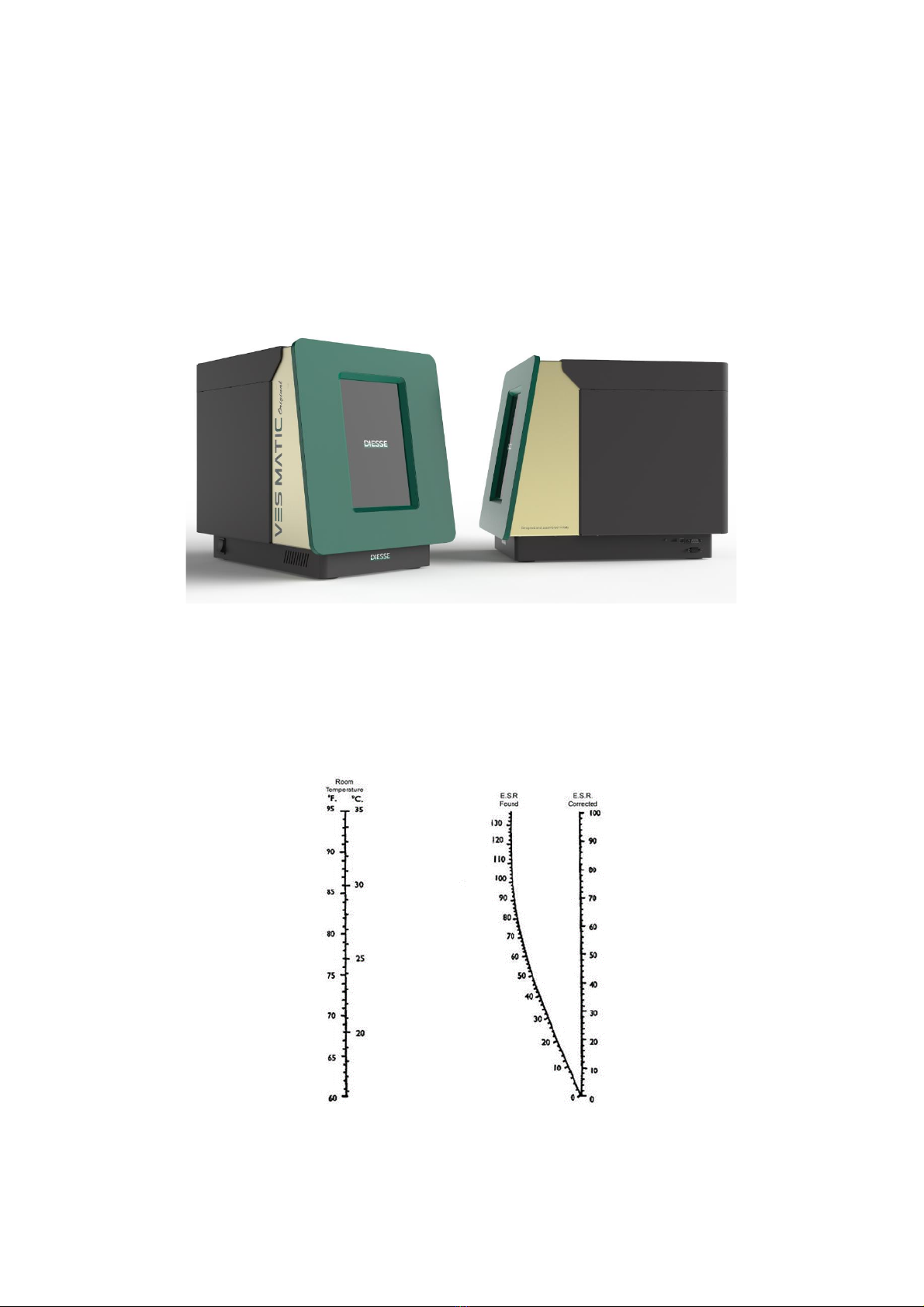
The VES MATIC Original (Figure 1) is an instrument designed to measure the quantitative
Erythrocyte Sedimentation Rate (ESR) with the modified Westergren method using
venous whole blood anticoagulated in citrate (Ref. 1,14-18). The instrument allows up to
30 blood samples to be analysed in random access and continuous loading mode.
The dedicated citrate tubes, compatible with the instrument, are those described in the
following table:
Tubes
Description
Codes
VES-TEC
Non-evacuated tubes to be
filled manually containing a
sodium citrate solution for
direct ESR determination.
Sample volume ≈ 1 ml (refer
to relevant Instructions for
Use).
10201/A
10214
VACU-TEC
Vacuum tubes for direct
blood sampling containing
a sodium citrate solution for
direct ESR determination.
Sample volume ≈ 1 ml (refer
to relevant Instructions for
Use).
10200
10200/W
10208
10600
10602
The instrument performs the sample analysis directly on the above-mentioned tubes,
designed for ESR examination: therefore, no sample transfer is necessary.
Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 11
The analysis is fully automated (mixing and reading), with random and continual loading
modes, in the latter case, by adding manually pre-mixed samples. The results are
comparable to those obtained with the Westergren method, providing results
equivalent to those obtained with the 1-hour Westergren method in only 30 minutes
and those obtained with the 2-hour Westergren method in only 60 minutes. (Ref. 1-10).
The maximum analytical output of the system is 60 results/hour.
Figure 1 –VES MATIC Original
The instrument, programmed to always work with the temperature correction activated,
reports results at a temperature of 18°C according to Manley's nomogram (Figure 2).
However, it is possible to de-select temperature correction according to laboratory
needs (Ref. 13).
Figure 2 - Manley’s nomogram

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 12
The blood collected in the relevant tubes is thoroughly mixed by the instrument; then,
the samples are left to settle for a set period until sedimentation occurs. Through a
digital sensor (optoelectronic unit), the instrument automatically measures the
erythrocyte sedimentation level: the results obtained are processed and automatically
printed or displayed on the screen or sent to the Host
Through a colour touch screen, it is possible to select the various functions of the
instrument's analysis cycle, which are:
•1-hour Westergren analysis: returns an ESR reading after 30 minutes of analysis,
according to the 1-hour Westergren method;
•2-hour Westergren analysis: returns two ESR readings (after 30 and 60 minutes)
and the corresponding Katz index after 60 minutes of analysis according to the
Westergren method, with readings after one hour and two hours;
•1-hour Westergren analysis in kinetics: returns an ESR reading after 30 minutes
according to the 1-hour Westergren method. At the end of the examination, the
graph showing the sedimentation kinetics of the sample during the analysis can
be displayed;
•2-hour Westergren analysis in kinetics: returns two ESR readings and the
corresponding Katz index after 60 minutes according to the Westergren method
with readings after one hour and two hours. At the end of the examination, the
graph showing the sedimentation kinetics of the sample during the analysis can
be displayed.
All results of analysed samples (and controls) are stored and can always be consulted
(and printed) through dedicated archives.
1.3 Clinical significance of ESR
The erythrocyte sedimentation rate test measures the distance travelled by red blood
cells over a certain period. In normal conditions, red blood cells tend to move apart
reciprocally due to the presence of negative electric charge from the numerous residues
of sialic acid present at a membrane glycoprotein level. When an inflammatory process
or tissue damage alters the protein composition of the plasma, with the production of
so-called “acute phase proteins”, the binding of these proteins (fibrinogen,
immunoglobulins) with the surface of the red blood cells alters the negative membrane
charge (Z-potential) and these can stack up to form so-called rouleaux.

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 13
These rouleaux cells then aggregate to form microspheres of a uniform radius, which
begin to settle when their density exceeds that of the plasma. The ESR value goes up in
all cases where there is an increase in acute phase proteins, in particular fibrinogen
(which is considered to account for 70% of the sedimentation phenomenon) and
immunoglobulins (which increase in the case of oncological/haematological diseases
and acute infections). The ESR is, therefore, an indirect and non-specific measure of an
inflammatory state and is increased in various pathological conditions such as
inflammatory diseases (infections, rheumatic diseases), a relative/absolute increase in
globulins (nephrotic syndrome, myeloma), tissue necrosis (myocardial infarction,
tumours). The ESR is useful for predicting the outcome prognosis of certain disorders
such as polymyalgia rheumatica, giant cell arteritis, rheumatoid arthritis and Hodgkins'
disease and can be used as a marker of treatment efficacy in several diseases such as
rheumatoid arthritis, vasculitis, collagenosis, and septic arthritis (Ref. 11, 24). The
erythrocyte sedimentation rate is usually higher in women than in men and is also
increased during pregnancy; it also tends to rise with age in both genders (Ref. 23).
1.4 Normal ESR values (Westergren citrated)
The international reference method for ESR measurement is the Westergren method,
performed on blood anticoagulated in citrate, with four parts blood to one part anti-
coagulant. The diluted blood is then aspirated inside a special, graduated, 2.5-mm
diameter pipette and kept upright. The erythrocyte sedimentation level is recorded after
one hour by measuring the distance between the lower side of the plasma meniscus
and the upper side of the sedimented red blood cells.
International guidelines indicate the following range as reference ESR values according
to the Westergren reference method*:
Normal 0-20mm/hr
* following CLSI Procedures for the Erythrocyte Sedimentation Rate Test; CLSI document
H02 (Ref. 25).
Reference values should be established locally in compliance with the laboratory’s
accrediting agencies. Refer to CLSI document C28-A2 for age and gender-specific
reference values (Ref. 26).
International guidelines identify 3 ranges of ESR: low (ESR <20 mm/hr), medium (20 <ESR
<60 mm / hr) and high (ESR> 60 mm/hr) (Ref. 14-18) regardless of gender and age. The
scientific literature indicates that the normal ESR value for ages under 50 is between 1

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 14
and 15 mm/h for men and between 1 and 25 mm/h for women. For older ages, these
values increase, standing between 1 and 20 mm/hr for men and between 1 and 30 mm/hr
for women. In pathological conditions, such values can increase up to 100 mm/hr or
higher.
These values are to be considered purely indicative and vary according to age and
gender, and geographical location. According to international guidelines, each
laboratory should determine its own normal ranges according to gender and age groups
(Ref. 26).
A physician must interpret the clinical significance of ESR values obtained
from abnormal samples, including but not limited to samples presenting
icterus, lipaemia, anaemia, low haemoglobin concentrations, haemolysis, or any other
pathological condition that may interfere with or prevent a clear reading of the
sedimentation. ESR testing performed on anomalous samples using manual or
automated methods are subject to a high degree of variability.
In the VES MATIC Original, these samples may be undetected or yield varying results; for
this reason, a visual inspection of the sample to verify the presence of a clear interface
between the plasma and sedimented cells at the conclusion of the test is recommended.
1.5 Material required for using the instrument
To operate the instrument, use
only
materials from the VES MATIC Original line
manufactured by DIESSE DIAGNOSTICA SENESE S.p.A. (Always read the instructions for use
which accompany each product before its use); any other part or accessory used in the
instrument may cause damage or incorrect results. The manufacturer, therefore, declines all
responsibility for damages deriving from inappropriate use.
1.6 Warnings
While the VES MATIC Original system provides a high level of safety in handling
biological samples, please take all necessary precautions when handling
potentially infected material.
All precautions required by law must therefore be taken. The waste material at the end
of the cycle must also be processed like any other material that qualifies as hospital
waste.

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 15
1.7 Personal protective equipment (PPE)
Normal use of the instrument does not require the user to come into contact with a
biological sample.
In any case, the user must wear personal protective equipment in compliance with the
laws of his/her country. PPE consist of at least:
▪Gloves
▪Safety glasses
▪Lab coat
1.8 Ordinary maintenance
The VES MATIC Original has been designed and built to require minimal
maintenance. Before any maintenance works:
•Turn off the instrument and disconnect it from the power supply
•Use suitable personal protective equipment (paragraph 1.7) during
operation
It is recommended to restart, turn off and on the instrument at the end of the day to
avoid memory overload issues of the VES MATIC Original.
1.8.1 Cleaning and decontamination
External cleaning is recommended for safety reasons.
The decontamination procedure must be carried out by the user in the event of leakage
of biological material, displacement of the instrument or when deemed necessary.
In the event of biological material leakage during the operating cycle, wear
personal protective equipment to clean the external surfaces of the instrument.
Decontamination procedure:
1. With the instrument turned off, remove any residue and/or spillage using a
disinfectant liquid used in the laboratory and allow it to dry (the recommended
disinfectant agent is Umonium® 38 Medical Spray). For the touch screen, use a
dry microfiber cloth.
2. Leave the instrument turned off for at least 1 hour before starting a new operating
cycle or performing any other intervention on the instrument.

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 16
For assistance with the inside of the instrument not easily accessible to the
operator, contact Technical Services.

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 17
2TECHNICAL DATA
2.1 Technical description
Figure 3 - Instrument Layout
The VES MATIC Original consists of a single body containing all the operating functions
necessary for analysing the sample.
Test Tube Plate
The instrument is fitted with a 3D-printed test tube plate with 30 slots arranged in two
concentric circles of 15 positions each that allow accommodating the samples.
Reading unit
A motor lifts the reading unit, which uses 2 optical sensors to verify the suitability of the
sample and detect the level at time zero and the end of sedimentation.
Sample detection
Sample detection takes place via the optical reading sensors after the cycle has started.
The instrument scans the test tube inserted in each position, verifying that it contains
an adequate volume of blood.

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 18
Sample identification
The sample is identified by an internal barcode reader that reads the code after the
sample is detected by the optical sensor. If not read automatically by the instrument, the
sample identification code can also be entered manually using a virtual keyboard or an
external barcode reader (accessory).
Acoustic warning
The function of the acoustic warning is to alert the operator during various stages of the
operating cycle or in the event of errors.
Temperature sensor
This sensor is used to measure the temperature and is fitted in the proximity of the test
tube plate. If the temperature is outside the operating range described in paragraph 9.1,
a message on the screen will signal the condition to the user.
Printer
The analysis results are printed at the end of each operating cycle or working session.
2.2 External connections of the instrument
The power connector is on the back of the instrument (Figure 4).
Figure 4 - Power connector
The “I “ [ON] / “O” [OFF] switch is on the left side of the instrument base (Figure
5).
Figure 5 - Power button

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 19
On the right side of the instrument base, the following are present:
•Two ports for connection to a Computer/HOST: one is Serial RS232 (9pin), the other
is USB standard-b.
•One port for connection to an external barcode reader (EXT. BC).
•One USB a-standard port for connecting to a USB mass storage device (usable for
software updates and exporting files) (Figure 6).
Figure 6 –Connectors for external supports
2.3 Software Update
Software updating is a simple, direct procedure:
•Download the software from www.diesse.it
•Save the contents of the “VM_Original_version_number” folder in a USB memory
device.
Note: depending on the update, the folder "VM_Original_version_number" may
contain:
a. one file: mcor.bin
b. two files: mcor.bin and System folder, which contains the graphic object
required for the instrument to function. In this case, the folder name is
“VM_Original_version_number_with_system”
c. three files: mcor.bin, System folder, and Help folder, which contains the files
necessary to update the instrument help.
Updating the Help is not an automatic procedure, but the user must use the
appropriate function in the Service. Refer to the Service Manual for
instructions.
•With the instrument switched off, insert the USB device into the appropriate port
(Figure 7)
•Power on the instrument and wait for a few seconds. The instrument will update
automatically.

Rev. 1.0 (09.2022) VES MATIC Original | USER MANUAL USER 20
Figure 7 - USB port
The version number is visible on the “Service” page (Figure 57).
2.4 Technical features
USE
Internal use
POWER SUPPLY
Europe: 230Vac@50Hz;
Usa/Canada: 110-120Vac@60Hz
Power output: 100VA
DIMENSIONS (mm)
310 x 460 x 403 (l x w x h)
WEIGHT
15 kg
TEMPERATURE
15-35°C (operating)
5-45°C (storage)
RELATIVE HUMIDITY
(RH)
20%-80% without condensation
ALTITUDE
Up to 2000 meters
NOISE LEVEL
Below 80 decibels
POLLUTION LEVEL
2 pollution degrees
MEASUREMENT
RANGE
1 - 140 mm/h
CENTRAL UNIT
ARM Cortex-M4 180 MHz Microprocessor
DISPLAY
10.1” vertical, wide
OPTICAL UNIT
2 pairs of optical elements
INTERFACES
USB Host; USB Client; 2x RS232
PROTECTION CLASS
I
SAFETY STANDARDS
EN 61010-1; EN 61010-2-101
EMC
EN 61326-1/EN 61326-2-6
Other manuals for VES MATIC Original
1
This manual suits for next models
1
Table of contents
Other Diesse Analytical Instrument manuals