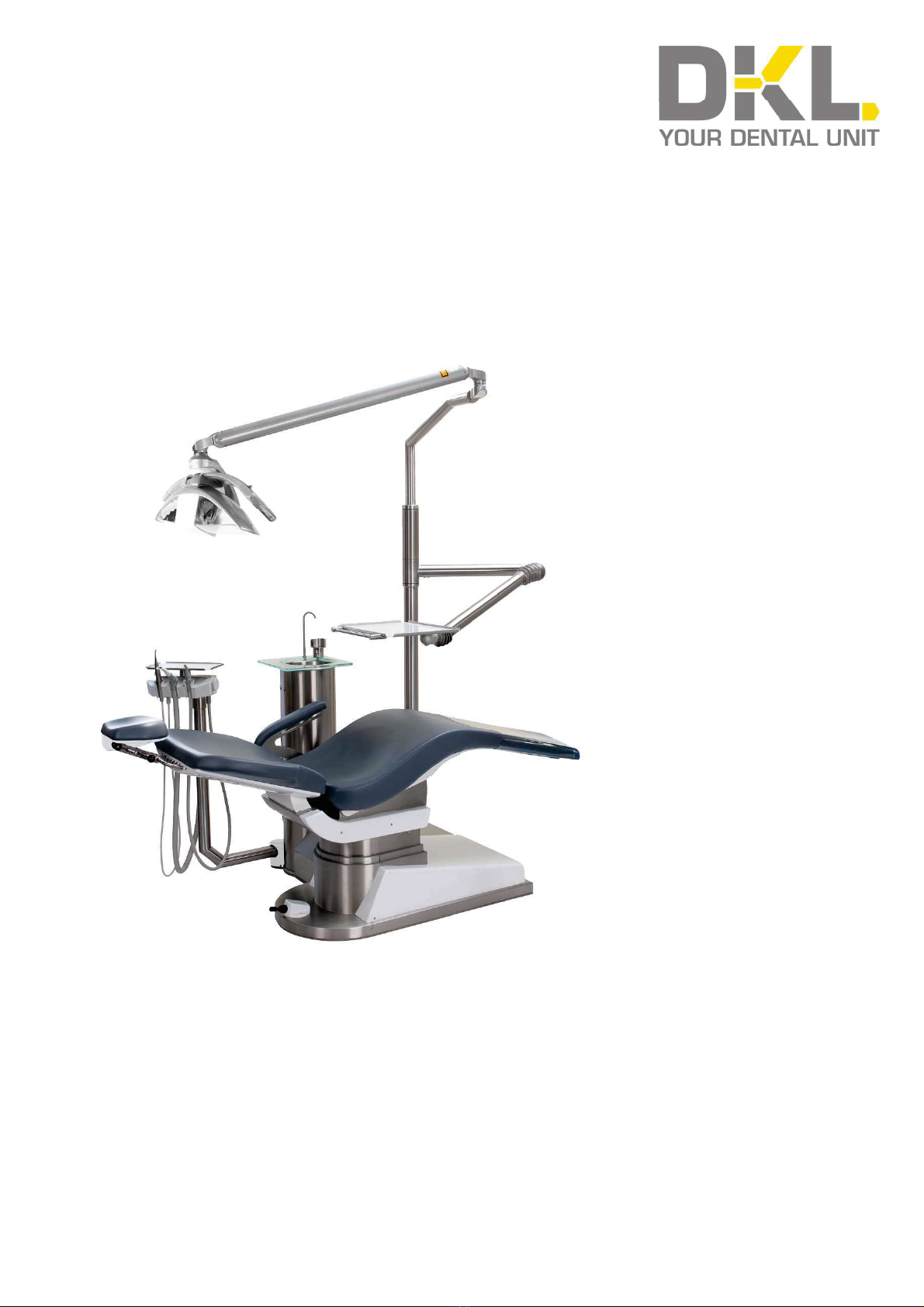
10
Media water
Water hardness 1.5 to 2.14 mmol/l = 8,4-12 dH
ph-value 6,5 to 8,5
Water filtration on site ≤ 100 µm
Water inflow Pipe 10x1mm, angle valve outlet 3/8“
Water connection above floor min. 40 mm, max. 60 mm
Water inlet pressure 2.0 to max. 6.0 bars
Water quality Cold water in accordance with local and national drinking
water regulations.
Minimum flow rate 3 l/min
Media air
Air inlet pressure max. 7 bars
Air consumption 80 Nl/min
On-site air filtration ≤ 100 particles size 1 - 5 μm referred to one m3of air
Oil content ≤ 0.5mg/m3,oil-free compressors; the compressor must suck
in hygienically perfect air.
Humidity Pressure dew point ≤ -20 °C at atmospheric pressure
Compressed air supply Pipe 10x1 mm, angle valve outlet 3/8“
Air connection above the floor min. 40 mm, max. 60 mm
Media Requirements
• Perform the installation according to the national installation requirements (e.g. EN 1717).
• For the reduction of microorganisms in the water supply pipe, please observe the following when laying this pipe to the
treatment unit:
- Avoid long stub lines to the treatment unit.
- Select the installation in such a way that other essential consumers (e.g. washbasin) are as far as possible
behind the connection of the treatment unit can be supplied from the same pipe.
- Avoid laying the hot water supply pipes in parallel.
• Recommendation: For the water supply of the treatment unit, install an angle valve with 2 outlets and 2 stop cocks. The
second outlet allows easy sampling of water for microbiological analysis.
Clean air and water pipes before installing the unit.
Chips and other foreign substances could be flushed or blown into the treatment unit.
Metal chips can interfere with the function of pneumatic components. Filters are clogged by foreign substances.
• When assembling, make sure that there are no chips or other foreign substances in the pipes.
• Flush the water pipes.
• Blow out the air ducts.
• Make sure that no further foreign substances get into the pipes and ducts after rinsing or blowing out.
Before the treatment unit is installed, the microbiologically perfect water quality of the domestic water supply
should be ensured and documented in the form of a microbial count.
Sampling and microbial count should be carried out by a competent laboratory.
Connection to the public drinking water supply
The treatment unit with a water separation unit complies with the requirements of EN 1717 (free outlet with separation
distance ≥ 20 mm) and the DVGW (German Technical and Scientific Association for Gas and Water). It is intrinsically safe
in accordance with worksheet W540 and therefore also meets the requirements of W270 and KTW (guideline for hygienic
assessment of organic materials in contact with drinking water).
When the treatment unit is equipped with a cuspidor, the bowl rinser ensures the free outlet with a separation distance
≥ 20 mm. When the treatment unit is equipped with a Bottle Care System, the spray supply of the instruments is separate
from the public water supply.