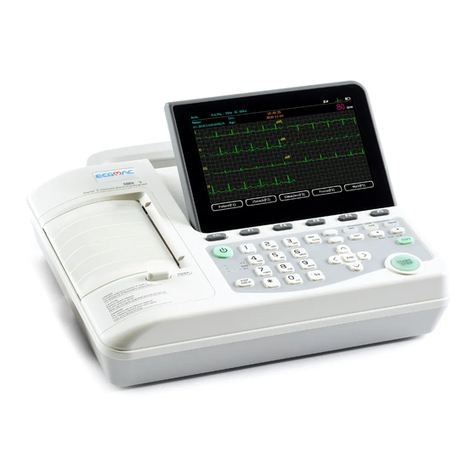
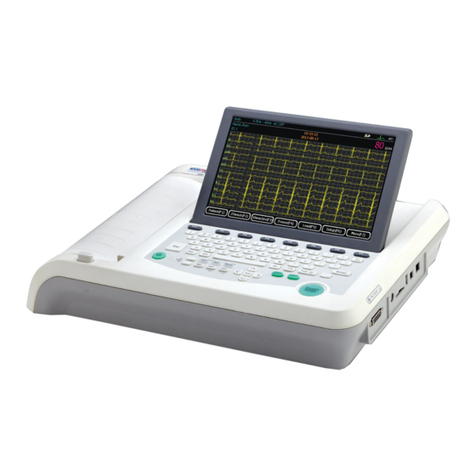
ECGMAC EM-301 User manual


Copyright ©2012 Shenzhen ECGMAC Medical Electronics Co., Ltd. All rights reserved.
Statement
Shenzhen ECGMAC Medical Electronics Co., Ltd (hereinafter called ECGMAC) assumes no
responsibility for any injury, or for any improper or illegal use of the product, that result from
failing to comply with this manual, ECGMAC assumes no consequence caused by the publication.
Any part of the manual shall not be reproduced, photocopied in any form without prior written
permission from ECGMAC.ECGMAC reserve the right to change, update, revise the product
design, specification and function at any time without notice.
Responsibility of the Manufacturer
ECGMAC only considers itself responsible for any effects on safety, reliability and performance of
the equipment if:
Assembly operations, extensions, re-adjustments, modifications or repairs are carried out by
personnel authorized by ECGMAC, and the electrical installation of the relevant room complies
with safety standards.


1
Contents
1 Safety Guidance.......................................................................................................... 3
1.1 Installation and storage ................................................................................................................................ 3
1.2 Before operation .......................................................................................................................................... 3
1.3 During operation.......................................................................................................................................... 3
1.4 After operation............................................................................................................................................. 4
1.5 EMC and relevant notes............................................................................................................................... 4
1.6 Device classification.................................................................................................................................... 4
1.7 Measurement requirement ........................................................................................................................... 5
1.8 Discarding the equipment............................................................................................................................ 5
1.9 Symbols ....................................................................................................................................................... 5
1.10 Recording paper......................................................................................................................................... 6
2. Safety Information..................................................................................................... 7
2.1 Safety Warning ............................................................................................................................................ 7
2.2 Warnings for rechargeable lithium battery................................................................................................... 8
2.3 General Notes .............................................................................................................................................. 9
2.4 Cleaning, Disinfection, Maintenance......................................................................................................... 11
2.5 Intended Use .............................................................................................................................................. 12
2.6 Contraindication ........................................................................................................................................ 12
3 Structure and principle ............................................................................................. 13
3.1 Product structure........................................................................................................................................ 13
3.2 Electrodes connection................................................................................................................................ 18
3.3 Patient cable connection ............................................................................................................................ 20
3.4 Power connection....................................................................................................................................... 21
3.5 Principle and Schematic diagram .............................................................................................................. 22
3.6 Features...................................................................................................................................................... 23
4. Operation Preparations ............................................................................................ 23
4.1 Applicable fields ........................................................................................................................................ 23
4.2 Environmental operating conditions.......................................................................................................... 23
4.3 Inspection before operation ....................................................................................................................... 23
4.4 Lead off indication..................................................................................................................................... 24
4.5 Battery Installation..................................................................................................................................... 25
4.6 Recording paper......................................................................................................................................... 25
4.7 Loading recording paper............................................................................................................................ 26
5 Operation Instructions .............................................................................................. 26
5.1 Startup and shutdown ................................................................................................................................ 26
5.2 The ECG main interface ............................................................................................................................ 27

2
5.3 Operation mode ......................................................................................................................................... 29
5.4 Leads setup ................................................................................................................................................ 36
5.5 Display setup ............................................................................................................................................. 37
5.6 System setting............................................................................................................................................ 38
6 Cleaning, Disinfection and Maintenance ................................................................. 46
6.1 Cleaning..................................................................................................................................................... 46
6.2 Disinfection ............................................................................................................................................... 46
6.3 Care and Maintenance ............................................................................................................................... 47
7 Common troubleshooting and solution .................................................................... 49
7.1 Some lead without waveform printout....................................................................................................... 49
7.2 Vertical breakpoint of printed waveform ................................................................................................... 49
7.3 Buttons on the control panel not working.................................................................................................. 50
7.4 AC interference.......................................................................................................................................... 50
7.5 EMG interference ...................................................................................................................................... 50
7.6 Baseline Drift............................................................................................................................................. 51
8 Warranty and after-sale service ................................................................................ 52
8.1 Warranty .................................................................................................................................................... 52
8.2 Customer service ....................................................................................................................................... 52
Appendix A Packaging and Accessories ..................................................................... 53
A.1 Packing ..................................................................................................................................................... 53
A.2 Caution...................................................................................................................................................... 53
Appendix B Product Performance............................................................................... 54
B.1 External Output......................................................................................................................................... 54
B.2 External DC signal Input........................................................................................................................... 54
Appendix C Specification ........................................................................................... 56
C.1 Technical Index ......................................................................................................................................... 56
C.2 Dimension and weight .............................................................................................................................. 58
C.3 Environment conditions ............................................................................................................................ 58
Appendix D Applied standards ................................................................................... 58
Appendix E EMC information .................................................................................... 60
Appendix F Manufacturer information ....................................................................... 63

3
1 Safety Guidance
This is provided for the use of qualified physicians or personnel professionally trained.
The operator is supposed to be familiar with the contents of this Operation Manual before
operation.
This is applicable for ECG signal acquisition, recording and analysis.
1.1 Installation and storage
zAvoid contact with water. Don’t install and store in the high air-pressure, humidity and
temperature, poor ventilation and dusty environment;
zPut the ECG in the stable place to avoid vibration;
zDon’t install and store it in the environment including sulphur, salt and soda and other
chemicals;
zBe sure there is no intense electromagnetic interference source around the equipment, such as
high-voltage cable, radiological equipment and magnetic resonance imaging equipment.
zMake sure the integrity of the external protective earth conductor in the room
1.2 Before operation
zCheck if the equipment is in good condition;
zCheck if the equipment is placed properly;
zCheck if all the lead wires are well connected and the equipment is properly grounded;
zWhen the instrument is used with other instruments, special attention should be paid to safety,
misdiagnosis and other problems;
zAll circuits which directly contact patients need to be inspected more carefully;
zThe voltage and frequency of the AC power must match the requirements on the user manual
and the battery capacity must be sufficient when battery is applied.
1.3 During operation
zDuring the operation, the doctors cannot leave alone the patients. Watch the patient carefully
and turn off the power or remove the electrodes if necessary to ensure the safety of patients;
zExcept electrodes, patients should not contact other parts of the instrument or other conductors

4
1.4 After operation
zReset all the function setup to the initial state and shut down the power;
zGently remove the electrodes, and do not harshly drag the wires;
zClean the instrument and accessories for the later use.
1.5 EMC and relevant notes
This instrument complies with IEC60601-1-2 standard about the medical electronic equipment
safety standards on electromagnetic compatibility. But in an electromagnetic environment
exceeding the limitation of IEC60601-1-2 standard, the instrument might suffer from harmful
interference and will not provide expected function or get worse performance. If the performance of
the instrument is degraded while working, please check and eliminate possible adverse effects
before any further use. This manual provides the following preventive procedures.
1.5.1 The influence of electromagnetic radiation
Mobile phone may affect the operation of this equipment. Once this equipment is used, be
sure to remind people to turn off mobile phones and small wireless devices.
1.5.2 The influence of Impact and electromagnetic waves
The high frequency noise made by other instruments may bring electromagnetic interference
to the instruments via entering AC outlet. Please identify the noise source and stop using the
corresponding equipment. If it can not be stopped then use de-noising equipment to reduce
the influence.
1.5.3 Electrostatic affect
The static electricity in a dry room may affect the operation of this instrument, especially in
the winter. Before using this instrument, humidify the air in the room, or discharge the static
electricity from cables or the ECG patients.
1.5.4 Impact of lightning
Nearby lightning may cause high-voltage surge. If worried about this issue, please disconnect
the AC power and use the battery power.
1.6 Device classification
zProtection against electric shock: Class I, Type CF internally powered
zProtection against ingress of hazardous liquid : Normal equipment (no protection against
ingress of hazardous liquid)
5
zCombustible gas safety: Not suitable for use under the presence of flammable gas
zMode of operation: Continuous
zEMC: Class B
1.7 Measurement requirement
ECG belongs to measurement instrument. The users are suggested to send the instrument to
authorized Measurement Institutes for verification at least once a year according to the state ECG
and EEG metrological verification regulations.
1.8 Discarding the equipment
Discard electronic materials, packing materials, battery and other declared waste according to the
local laws. Support the sorting recycling work of the local administration.
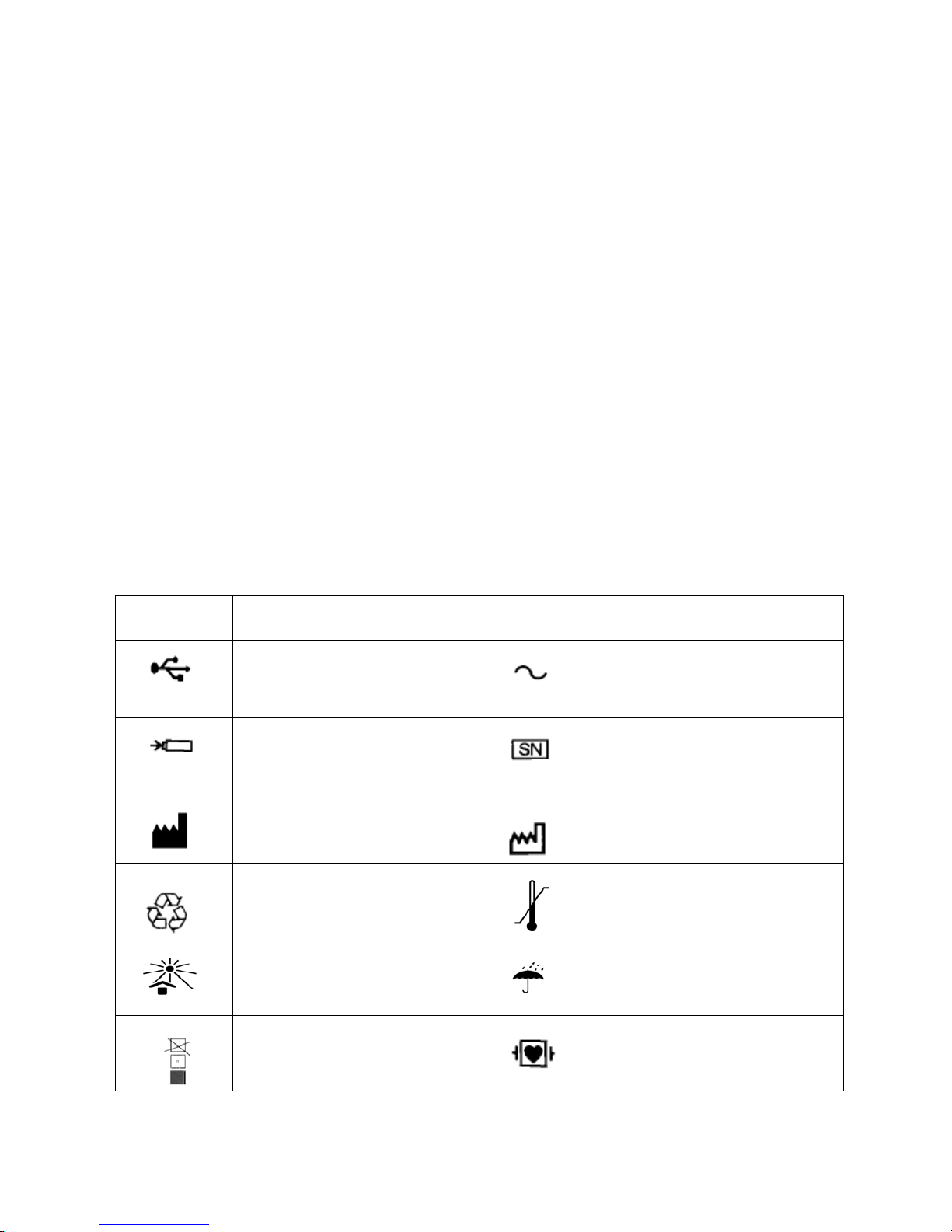
1.9 Symbols
Symbols Function Symbols Function
USB
Alternating current
Battery recharging indicator
Product serial number
Manufacturer
Date of manufacture
Recycle
Storage temperature range
Avoid direct light
Keep dry
Tier limitation
Equipment of type CF with
defibrillator proof

6
Battery indicator
Note (general warning): the
information you should know to
avoid possible damage to patients
or operators.
Note: the information you
should know to avoid possible
damage to the equipment
Potential equalization
Remark:
Note (general warning): the information you should know to avoid possible damage to
patients or operators.
Note: the information you should know to avoid possible damage to the equipment
1.10 Recording paper
To guarantee the record quality of the ECG waveforms, please use the high speed thermal paper
supplied or appointed by ECGMAC. Other paper may shorten print head’s life. And the deteriorated
print head may lead to illegible ECG record and block the advance of paper etc
Please do pay attention to the following aspects:
(1) Never use wax-coated recording paper. Otherwise wax may stick to the heater of the print head
and damage it;
(2) Record paper should be stored in dry and cool area, avoiding excessive temperature,humidity
and sunshine;
(3) Avoid exposure to fluorescent for a long time, it will influence the recording quality;
(4) Be sure that there is no polyvinyl chloride or other chemicals in the storage environment, which
will lead to color change of the paper;
(5) Do not overlap the recording paper, otherwise the ECG record may trans-print each other;
(6) Pay attention to the size of the paper. Improper size may damage the printer head or Roller shaft.

7
2. Safety Information
2.1 Safety Warning
Use three core power cord and protective grounding socket when mains supply is applied, in
order not to cause electric shock to patient and operator.
Be sure that the installation room has stable power supply system which is reliable and
grounding.
When the system is not complete and reliable, cut off the A/C power and use internal D/C
supply directly
Do not use the instrument near anesthetic gases, oxygen, hydrogen or other flammable and
corrosive chemicals.
Do not use the instrument near high-voltage, high- static, X ray, ultrasonic, electrosurgical
equipment and other strong magnetic wave environment.
Only qualified service engineers can install this equipment. And only service engineers
authorized by ECGMAC can open the shell case.
Auxiliary equipments connected to the digital and analog interfaces must be certified
according to IEC standards (e.g. IEC950 for data processing equipment and IEC60601-1 for
medical equipment). Therefore anybody, who connects additional equipment to the signal input or
output connector to configure a medical system, must make sure that it complies with the
requirements of the valid version of the system standard IEC60601-1-1. If any questions, please
consult our service department or the local agent on your side.
When defibrillator is used simultaneously with the instrument, the operator should not
touch patient, bed, table or instrument. All the electrodes (whether connected to the patient or not)
and the patient do not need to be grounded. When the instrument is operated simultaneously with
defibrillator or other electrical stimulation equipment, it is recommended to use disposable plate
chest electrodes to avoid skin burns by metal electrodes.

8
Be careful when patient is connected to multiple equipments, the total leakage current
might cause the injury. Only type I equipment compliant with GB9706 is allowed to connect with
this instrument, when it is connected with other equipments. Meanwhile, reliable connection
between the potential equalization needs to be carefully considered. After the connection, the
users must measure the total leakage current by themselves to determine whether it meets the
requirements or usage condition.
The pacemaker (if installed) may influence the accuracy and analysis result. Under this
situation, it is suggested that the doctors should identify and analyze it according to the waveform.
To prevent burns, high frequency electric knife contact point must be kept away from the
electrodes. If necessary, plate electrodes can be chosen. Its larger contact area can limit
high-frequency current density to an acceptable range.
The total leakage current should never exceed leakage current limits while several other
instruments are used at the same time.
The operator should not touch patient, patient bed, table or instrument when it use
simultaneous with the defibrillator or pacemaker.
Please use the patient cable and other accessories supplied by ECGMAC. Otherwise, it
will affect the performance and function, even damage the ECG.
Be sure that all electrodes have been connected to the correct position of human body
before operation. Avoid electrodes (including neutral electrodes) and patient to be contacted with
any conducting parts or earth.
2.2 Warnings for rechargeable lithium battery
Improper operation might cause the rechargeable lithium battery to become hot, ignited, exploded,
and it may lead to declination of battery capacity. It is necessary to read the Operation Manual
carefully and pay more attention to warning messages.
Danger of explosion- Do not reverse the anode and cathode when connecting the battery.

9
Do not use the battery near fire or place over 60℃. Don’t heat or splash the battery. Do not
throw the battery into fire or water.
Do not ruin the battery by hammering, beating, chiseling a metal into it or other else, which
will cause the battery to be deformation, heat, to smoke, burn and other danger.
When leakage or foul smell found, stop using the battery immediately. If the leakage liquid
gets to your skin or cloth, cleanse it when clean water at once. If the leakage liquid gets into your
eyes, do not wipe them. Irrigate them with clean water first and go to see a doctor immediately.
Opening the battery cover, disassembling or replacing battery should be done according to
the Operation Manual and only battery of same model and specification provided by the
manufacturer should be used.
When the shelf life of battery is due, or foul smell and leakage has been found, stop using it,
and contact the manufacturer or local distributor for disposal or dispose the battery according to
local regulations.
Do not pull in or take out the battery when the equipment is powered on, which will cause
white screen, crash, etc.
Take out the battery when not using the equipment for long time
2.3 General Notes
In order to record ECG data accurately, the instrument should be placed in a horizontal table
to avoid excessive vibration and shock during movement. The environment should be quiet and
comfortable.
Avoid liquid splash and excessive temperature. The temperature must be kept between 5℃
to 40℃while working.
Do not use the equipment in dusty environment with poor ventilation or in the presence of
corrosive, such as salt, sulphur and chemicals.

10
Be sure that there is no intense electromagnetic interference source around the equipment,
such as radio transmitter or mobile phone etc. Attention: large medical electrical equipment such
as electrosurgical equipment, radiological equipment and magnetic resonance imaging equipment
etc. are likely to bring electromagnetic interference.
Check the main unit and its accessories carefully before operating the ECG for patients.
Replacement should be taken if there is any evident defectiveness or aging symptom which may
impair the safety or performance.
The frequency and voltage of the AC power should conform to it requires. This ECG uses
mains supply and built-in rechargeable lithium battery. For mains supply, rated voltage: 220V,
frequency: 50Hz; power: 50VA. For battery, rated voltage: 14.8V, capacity: 4400mAh, power:
50VA.When using, please follow the requests to supply power.
Safety test should be applied on the instrument regularly (at least once every two years),
tests should include
a) Check if the instrument and accessories has mechanical and functional damage;
b) Check if the safety ID is broken;
c) Check the fuse whether it fulfills the requirement the rated current and short circuit
characteristic
d) Check the function of the instrument according to its operation manual
e) Perform the following safety test according to IEC60601-1 standard:
Protection ground impedance, Limit Value 0.1Ω.
Earth leakage current, Limit Value: NC 500uA, SFC 1000uA.
Patient current leakage, Limit Value: 10uA. (Type CF equipment)
When AC power is applied, limit value patient current leakage is 50 uA (Type CF equipment) in
single trouble status. The test results have to be recorded and performed by the authorized staff.
If the equipment failed in any of the tests, it has to be repaired.
The equipment and reusable accessories can be sent back to the manufacturer for
recycling or proper disposal after their useful lives.
If any accident happens during use, please turn off the ECG immediately.

11
2.4 Cleaning, Disinfection, Maintenance
Turn off the main unit and remove power supply cable and patient cable before cleaning and
disinfection.
Prevent the detergent from seeping into the main unit while cleaning. Do not immerse the
main unit or patient cables into liquid under any circumstances.
Do not clean the unit and accessories with abrasive fabric and avoid scratching the
electrodes.
Be sure no cleanser remains on the unit, patient cable or electrodes.
Disinfection, if required, can not be done with high temperature, autoclaving or radiation.
Do not use chloric disinfectant such as chloride and sodium hypochlorite etc.
Maintenance and repair should be applied on the main units and accessories regularly (at
least once every half year)
ECG machines are classified into Measuring Instrument, so users should send it to Official
Measurement Administrative to test and certify according to national metrological calibration
regulation of electro-cardiograph and electroencephalogram machine every year.
The instrument signal input/output connector (when needed) must be connected with Class I
equipment which is GB9706.1-compliant, and the total leakage current should be tested to be
available by users themselves.
Electrical schematic diagram and parts lists are only available to qualified repair stations or
staff of ECGMAC authorized.

12
2.5 Intended Use
The intended use is to acquire ECG signals from human through body surface ECG electrodes. The
ECG recorded by the electrocardiograph can help users to analyze and diagnose heart disease.
2.6 Contraindication
Absolute Contraindication
1. Acute myocardial infarction (within 2days)
2. High risk unstable angina
3. Hemodynamic disorder caused by uncontrolled arrhythmia
4. Active endocarditis
5. Symptomatic severe aortic stenosis
6. Decompensated symptomatic heart failure
7. Acute pulmonary embolus or pulmonary infarction
8. Acute noncardiac disorder that may affect exercise performance or be aggravated by exercise (eg,
infection, renal failure, thyrotoxicosis)
9. Acute Myocarditis or Pericarditis
10. Serious Physical Disability which disable to make safe and effective test
11. Without acquiring patient’s permission
Relative contraindications
1.Left main coronary stenosis or its equivalent
2.Moderate stenotic valvular heart disease
3.Electrolyte abnormalities
4.Tachyarrhythmias or bradyarrhythmias
5.Atrial fibrillation with uncontrolled ventricular rate
6.Hypertrophic Cardiomyopathy
7.Patients can not cooperate because of mental impairment
8.High-degree AV block
13
3 Structure and principle
3.1 Product structure
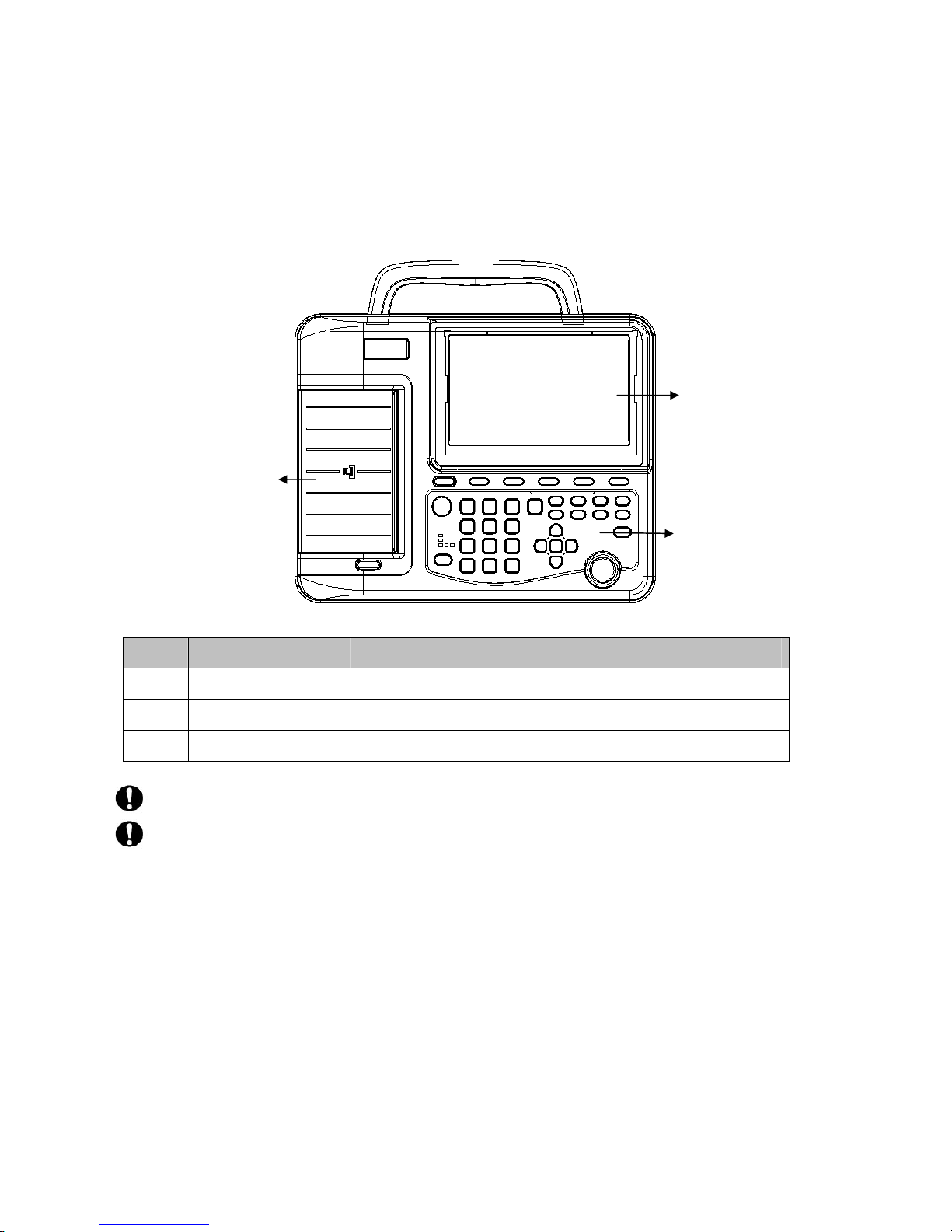
3.1.1Top view
Sign Name Description
A Recorder Load recording paper, print ECG report
B LCD screen Display operation interface and contents
C Keyboard Function buttons, input of numbers and letters
The LCD screen can be damaged if place heavy object on it or hit it.
Please fold the screen to prevent accidental damage after use.
B
A
C
14
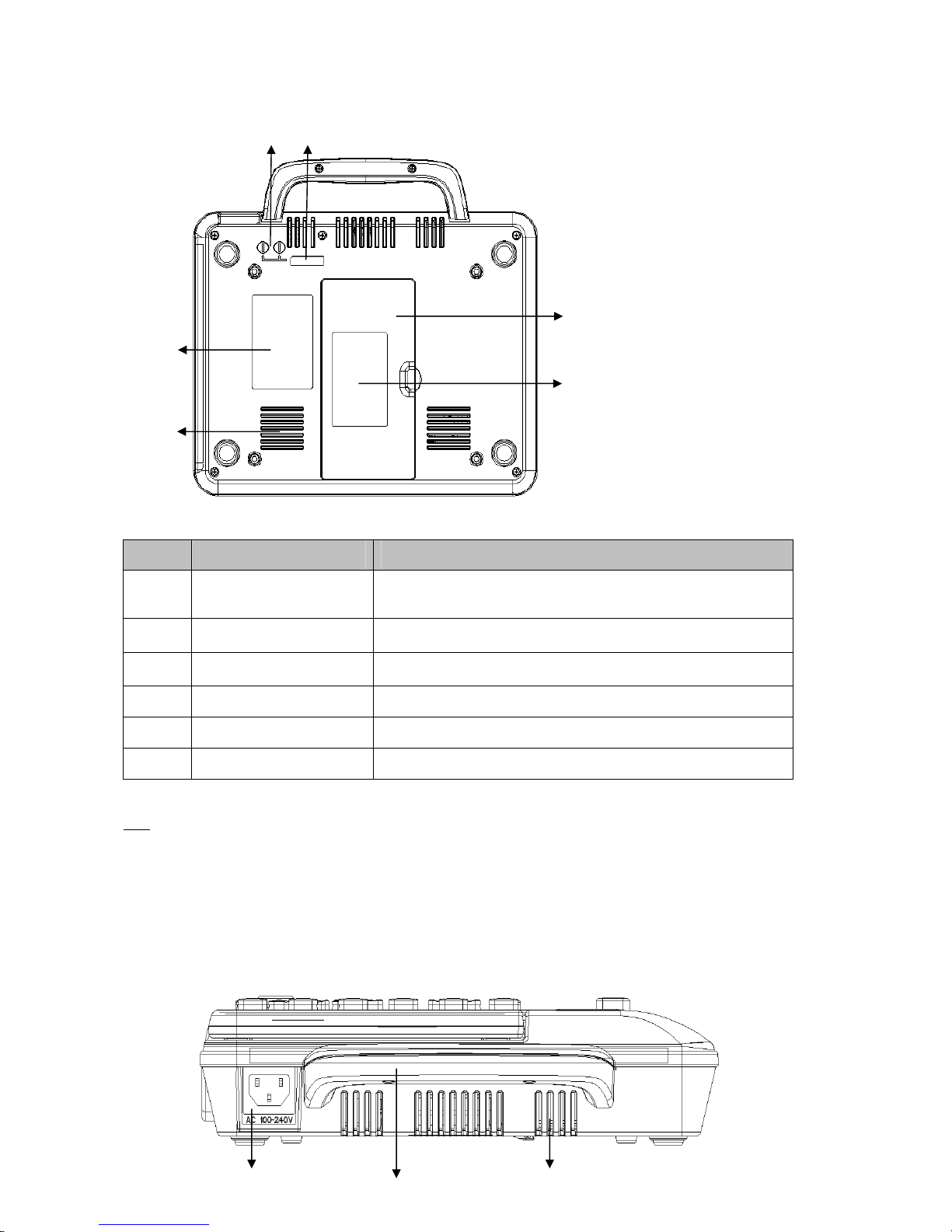
3.1.2 Bottom Panel
Signal Name Function description
A Battery compartment Lithium battery installed inside
B Battery label Battery group label
C Vents Internal heat dissipation channel
D Product label Product information label
E Fuse label Fuse specification label
F Fuse compartment AC fuse installation
1)Battery compartment
The rated output voltage and capacity of built-in rechargeable lithium batteries are as follow:
Rated output voltage: 14.8V
Rated capacity: 4400mAh
3.1.3 Rear View
B
A C
F E
C
A
B
D
15
Sign Name Function description
A AC power socket Connect with AC power cord
B Vents Internal cooling channels
C Handle Easy to carry
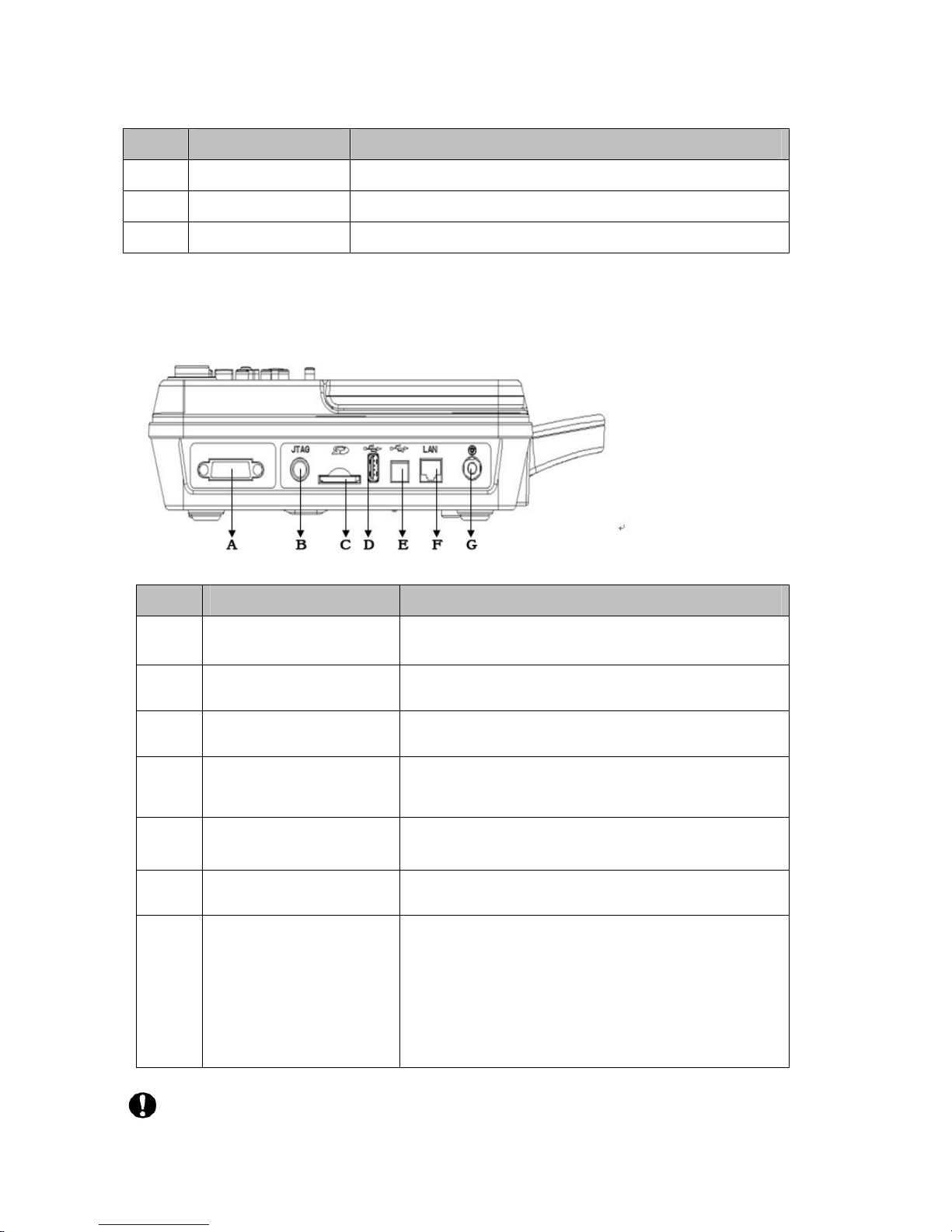
3.1.4 Side view
Sign Name Function description
A Patient cable socket Connect patient cable.
B Test interface Testing by manufacturer
C SD card slot For SD card insert
D USB Slave Port Standard USB 2.0 port to connect external printer
support PCL6
E USB Master Port Standard USB 2.0 port to connect PC
F LAN socket Standard LAN port, connect with network cable
G Potential equalization
When need to use potential equalization grounding
cable to protect the security of electricity, use
grounding cable to connect this potential
equalization terminal with the grounding cable
which is already connected to the walls
Test interface B list above is for manufacturer use only

16
3.1.5 Keyboard and its functions
Sign Button name Function description
A Numbers and letters input Enter numbers, letters and signs
B ESC Cancel operation
C Function buttons Select screen menu functions
D Backspace Delete the character to the left of the cursor
E Input method Choose the input methods: English / Numbers
F Five-way navigation buttons Up, down, left, right and action.
G Copy button Copy the last ECG signals when the system
works in automatic model.
H ON/OFF Turn on or off the ECG.
I Reset Make lead input fast and stable and reset lead
print output
J Recording mode Select recording modes: manual, automatic and

17
rhythm.
K Lead Select Lead switch in manual mode.
L START/STOP Start and stop print
M Sleep/wake up System enters sleep mode or back to work
mode
3.1.6 Patient Cable Socket and definition for plug pins
Applied part of type CF with defibrillator proof
Definition of corresponding pins:
Pin Signal Pin Signal Pin Signal
1 C2 (input) 6 SH 11 F (input)
2 C3 (input) 7 NC 12 NC
3 C4 (input) 8 NC 13 C1 (input)
4 C5 (input) 9 R(input) 14 NC
5 C6 (input) 10 L(input) 15 N or RF (input)
3.1.7 Patient cable
Patient cable includes main cable and lead wires. The lead wires include 6 chest lead wires
Lead Wires
Electrode Connectors
Main Cable
Screw
Connector
This manual suits for next models
1
Table of contents
Other ECGMAC Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Acteon
Acteon XMIND TRIUM Operator's manual
Siemens
Siemens SOMATOM Spirit Operator's manual

3A HEALTH CARE
3A HEALTH CARE MINIASPEED BATTERY PRO instruction manual

Breville
Breville Sommelier BWD600 Instruction book
Netatmo
Netatmo June manual

GLAXOSMITHKLINE
GLAXOSMITHKLINE Seretide 100 Diskus Instructions for use