EGi GES 400 MR Series User manual

GES 400 MR Series User Manual • 09-15-14
Electrical Geodesics, Inc.
500 E 4th Avenue, Suite 200
Eugene, OR 97401
USA
www.egi.com
GES 400 MR Series
MR Conditional Systems
User Manual
0297

2GES 400 MR Series User Manual | 8100401-55 •2015-07-22
Copyright © 2013–2015 by Electrical Geodesics, Inc.
All rights reserved.
The information in this document is
subject to change without notice.
Electrical Geodesics, Inc. (EGI) makes no
warranty or representation, either
express or implied, with respect to this
manual, its quality, accuracy,
merchantability, or fitness for a particular
purpose. In no event will Electrical Geodesics be liable for direct, indirect,
special, incidental, or consequential damages resulting from any defect or
inaccuracy in this manual, even if advised of the possibility of such damage.
Electrical Geodesics, Inc. (EGI)
500 E 4th Avenue, Suite 200
Eugene, OR 97401
USA
+1.541.687.7962 (tel)
+1.541.687.7963 (fax)
www.egi.com
European Union (EU)
Authorized Representative
Gerhard Frömel
MPS
Medical Product Service GmbH
Borngasse 20
35619 Braunfels
Germany
011 49 6442 962073 (tel)
011 49 6442 962074 (fax)
IMPORTANT NOTICE: The GES 400 MR and GES 410 MR products are sold
for strictly research purposes and are not medical devices in the United
States. As such these products cannot be used for medical purposes such
as the diagnosis, treatment, cure, mitigation, or prevention of diseases.
The GES 400 MR and GES 410 MR products are CE marked in conformity
with the European Medical Device Directive. Information on clearance
status in other countries is available upon request.

GES 400 MR Series User Manual | 8100401-55 •2015-07-22 3
GES 400 MR Series
MR Conditional Systems
User Manual

4GES 400 MR Series User Manual | 8100401-55 •2015-07-22

GES 400 MR Series User Manual | 8100401-55 •2015-07-22 5
Contents
Preface 7
1.Safety and Use Conditions 13
1.1Intended Use 13
1.2Features 14
1.3Conditions for Use 15
1.4Safety Warnings 20
2.FICS 29
2.1Overview and Specifications 29
2.2Physical Connections 31
2.3Removing/Inserting the Amplifier 34
3.
Net Amps Amplifiers
37
3.1Overview and Specifications 37
3.2Anti-aliasing Filter Effects on EEG Timing 38
3.3Physical Connections 40
4.Other GES Components 43
4.1HydroCel GSN MR Nets 43
4.2Net Amps Clock Sync 44
4.3Net Station and DAC 48
4.4Ethernet Switch 48
4.5GES External Power Supply 49
4.6Isolation Transformer 49

6GES 400 MR Series User Manual | 8100401-55 •2015-07-22
5.System Configurations 53
5.1EEG-fMRI 54
5.2EEG-fMRI with ERP 55
Appendix A: EMC Declarations 57

GES 400 MR Series User Manual | 8100401-55 •2015-07-22 7
Preface
elcome to the MR-conditional Geodesic EEG System™ 400 MR
(GES MR) Series systems from Electrical Geodesics, Inc. (EGI).
GES MR systems work within the magnetic resonance (MR)
environment to allow the simultaneous acquisition of electroenceph-
alographic (EEG) and functional magnetic resonance imaging (fMRI)
data. The components of GES MR systems, including the HydroCel
Geodesic Sensor Nets™ (HC GSNs, or Nets) and the Net Amps™ (NA)
amplifiers, have been specifically designed to significantly reduce the
EEG and MRI systems’ impact on each other by minimizing and
attenuating contrary signals and artifacts.
CAUTION: Only MR-trained personnel should work with this
equipment and only equipment labeled MR safe or MR conditional
should enter the specified MR environment. Adhere to all precautions
given in the “Safety and Use Conditions” chapter.
For all safety and use conditions for using the GES system outside of the MR
environment, refer to the GES 400 series manual (8100400).
GES MR System Models
Table P-1. Current models of the GES MR system
System Amplifier Sampling
Rate* Nets
GES 400 MR Net Amps 400
(NA 400) 8 Ksps all HC GSN MRs
GES 410 MR Net Amps 410
(NA 410) 20 Ksps all HC GSN MRs
*Sampling rates are software dependent. Net Station allows 1 Ksps and Amp Server Pro SDK facilitates
up to the full sampling rate capability of the amplifiers.
W

8GES 400 MR Series User Manual | 8100401-55 •2015-07-22
Table P-2. Parts list for typical GES MR systems
Part Qty Mfr Mfr P/N EGI P/N
Amplifier
Net Amps 400
(NA 400)
1 EGI
4608880 (256 chs)
4606168 (128 chs)
4606358 (64 chs)
4603285 (32 chs)
Net Amps 410
(NA 410)
4608884 (256 chs)
4606172 (128 chs)
4606362 (64 chs)
4603289 (32 chs)
Other system components
Field Isolation
Containment System
(FICS) enclosure
1 EGI 4609069 (256 chs)
4602986 (128 chs)
HC GSN MR 1 EGI Refer to the HC GSN
manual
Net Station (NS)
software
(includes the MR Artifact
Removal waveform tool)
1 EGI 3104200
HASP key 1 Safenet YWRGC 6158560
Net Amps Clock Sync 1 EGI 4608295
Ethernet switch 1 Black Box LGB2008A-R2 6156363
Isolation transformer 1 Toroid ISB-060M 6156331
GES external power
supply 1 EGI 4603986
Documentation
GES MR manual 1 EGI 8100401
NS manual 1 EGI version dependent
HC GSN manual 1 EGI version dependent
EEG/fMRI placard 1 EGI 8402100
Optional application
GeoSource 1 EGI 4602001
Note: This parts list assumes a typical system. Your system components
may differ, if you have upgraded or expanded your system.

GES 400 MR Series User Manual | 8100401-55 •2015-07-22 9
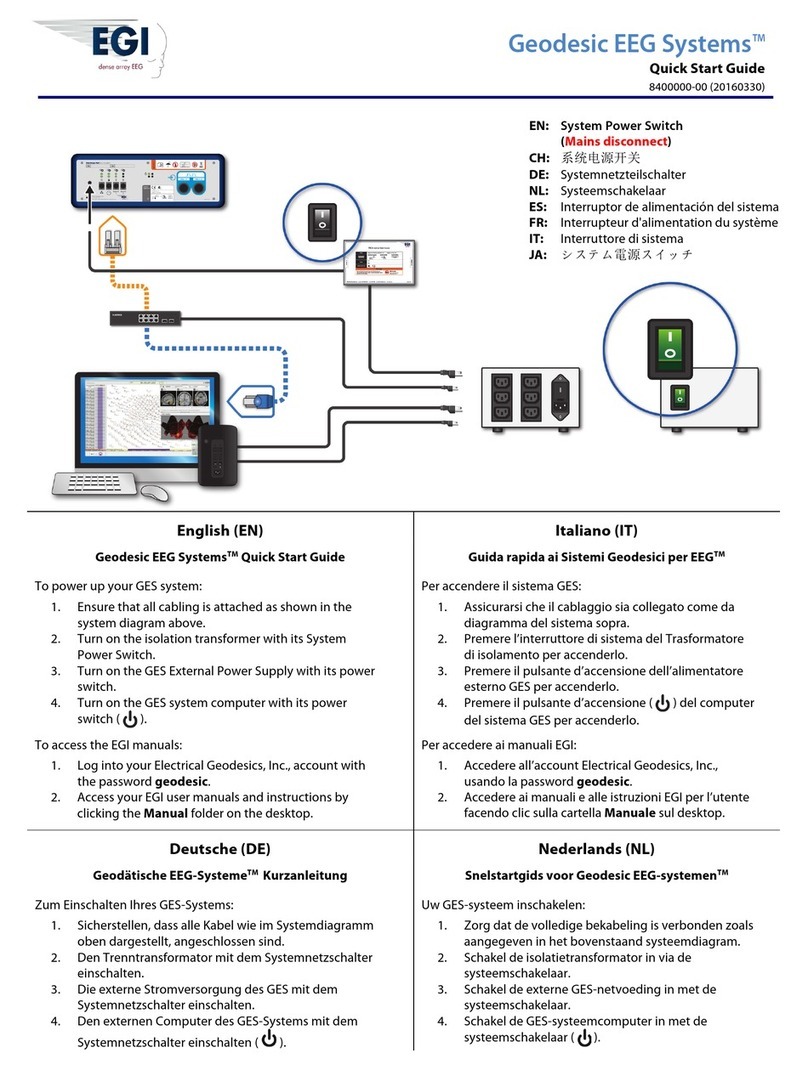
Typical GES MR System
GES MR systems operate within the MR environment to acquire
simultaneous EEG and fMRI data.
Figure P-1. Core components of a typical EEG-fMRI GES MR system

10 GES 400 MR Series User Manual | 8100401-55 •2015-07-22
About This Manual
This manual provides concise information for using the GES MR
system to display and record EEG data within the MR environment.
It assumes a working proficiency with EEG and computer systems.
Note: The term patient is used to refer to subjects, participants, or patients.
An EGI support or authorized engineer will install and configure your EGI
system, including all connections required for its operation. At the time of initial
installation, the EGI support or authorized engineer will also train relevant staff
in its operation. At any time you have additional questions or wish retraining,
contact EGI Technical Support (Table P-3).
Typographic Conventions
•Italics are used for definitions or newly introduced terms.
•Boldface italics are used for important concepts or for
special emphasis.
•Boldface is used for command paths (for example, File > Open).
Warnings, Cautions, and Notes
The following are used to convey important information:
WARNING: Warnings provide important information that, if
unheeded, could result in serious physical injury, death, or
equipment damage.
CAUTION: Cautions provide important information that, if
unheeded, could hinder the use of a product, feature, or
procedure, or result in physical injury or equipment failure.
Note: Notes provide clarifying information about a product, feature, or
procedure.

GES 400 MR Series User Manual | 8100401-55 •2015-07-22 11
Support, Repairs, and Documentation
If you have a question, please:
•For urgent issues during acquisition, contact EGI immediately.
•For nonurgent issues, do the following before contacting EGI:
–Isolate the problem. Try to repeat and define the
problem.
–Document the problem. Carefully record the
sequential details of the problem.
–Report the defined problem. Contact EGI.
Table P-3. EGI contact information
EGI Technical Support web page www.egi.com/support
Telephone +1.541.687.7962
Fax +1.541.687.7963
Address Electrical Geodesics, Inc. (EGI)
500 E 4th Avenue, Suite 200
Eugene, OR 97401
USA

12 GES 400 MR Series User Manual | 8100401-55 •2015-07-22

GES 400 MR Series User Manual | 8100401-55 •2015-07-22 13
1. Safety and Use
Conditions
For all safety and use conditions for using the GES system outside of the MR
environment, refer to the GES 400 series manual (8100400).
Do not operate your GES MR system until you are fully trained and
understand all warnings, cautions, and conditions for use provided
in EGI’s manuals for the components of your GES system. If you
have any questions, contact EGI Technical Support (Table P-3).
WARNING: All EGI system components must be installed and
configured by an EGI support or authorized engineer. Deviating
from the supported configuration or running the system with
non-EGI-approved components attached can cause hazards or
unexpected performance.
Note that the information in this manual is subject to change, without notice. The
manufacturer declines responsibility for the safety, reliability, and performance of
EGI system components, if not used in compliance with EGI documentation.
1.1 Intended Use
Geodesic EEG System™ 400 MR Series systems are intended to
measure and record the electrical activity of the brain. They can
be used on adults, children, and infants.
IMPORTANT NOTICE: The GES 400 MR and GES 410 MR products are sold
for strictly research purposes and are not medical devices in the United

14 GES 400 MR Series User Manual | 8100401-55 •2015-07-22
States. As such these products cannot be used for medical purposes such
as the diagnosis, treatment, cure, mitigation, or prevention of diseases.
The GES 400 MR and GES 410 MR products are CE marked in conformity
with the European Medical Device Directive. Information on clearance
status in other countries is available upon request.
1.2 Features
•ADAPT™ technology for on-board computing and
remote updates
•Dual-purpose Net Amps amplifiers usable both
inside and outside the MR environment
•Nonmagnetic, battery operated FICS amplifier
subsystem usable inside the MR imaging room
•Two ECG channels to facilitate ballistocardiogram
attenuation
•Data acquisition synchronized with MRI gradient pulses
•Easy application of HC GSN MR Nets with saline
solution
•HC GSN MR Net electrodes with internal SAR
mitigation/protection
•24 bit A/D conversion
•Fiber optic signal input and output for optimal
digital bandwidth and safety isolation, including:
•Ethernet data and control with Net Station
•Proprietary digital I/O, MR clock, and TR sync
•Vertex referenced inputs
•4 kV patient isolation to mains ground and
1.5 kV isolation mains to mains ground
•Choice of sampling rates (see Table P-1)
•Full range of channel counts (32 to 256)

GES 400 MR Series User Manual | 8100401-55 •2015-07-22 15
1.3 Conditions for Use
1.3.1 Environmental Conditions for Transport
and Storage
Table 1-1. Environmental conditions for the transport and storage of GES MR components
Shipping temperature 0˚ to 47˚C (32˚ to 116˚F)
Storage temperature -10˚ to 50˚C (14˚ to 122˚F)
Shipping humidity 5% to 95% noncondensing
Shipping altitude 11,500 m (37,700 ft) maximum
1.3.2 Environmental Conditions for Use
For EMC declarations, see Appendix A.
Table 1-2. Environmental conditions for the use of GES MR components
Operating temperature 10˚ to 35˚C (50˚ to 95˚F)
Relative operating humidity 5% to 95% noncondensing
Operating altitude 3,000 m (9,842 ft) maximum
1.3.3 Site Requirements for Location and Use
Table 1-3. Site requirements for the location and use of GES MR components
Location For indoor use only
Surface Area 102 x 150 cm (40 x 59 in.)
Ventilation Clearance 7.62 cm (3 in.)

16 GES 400 MR Series User Manual | 8100401-55 •2015-07-22
1.3.4 System Power
Table 1-4. Nominal power values for GES MR components
Device Rated Power Dissipation (watts)
FICS 9
NA 400 series amplifier 30
Mac Pro desktop computer 1,150
Mac 27-inch display 250
Dell computer 500
Dell 17-inch display 200
1.3.5 GES MR Electrical Specifications
Table 1-5. Electrical specifications for GES MRs
Maximum input signal amplitude ±200 mV
System input bandwidth 0 to 285 Hz (3 dB)
System dynamic range ≥115 dB
1.3.6 System Contraindictions
No contraindictions for the use of GES MR systems are known to exist.

GES 400 MR Series User Manual | 8100401-55 •2015-07-22 17
1.3.7 Certifications and Classifications
For all current EGI regulatory certifications, including CE declarations, go to
www.egi.com or contact EGI Technical Support (Table P-3).
This medical equipment is certified to the following:
•CAN/CSA C22.2 No 601.1-M90 (Safety of Medical Electrical
Equipment, Part I: General Requirements for Safety)
•CSA 601.1 Supplement 1:1994 (Requirements for Safety)
•CSA 601.1 Amendment 2:1998
•CAN/CSA C22.2 No 60601-2-26:04 (Particular
Requirements for the Safety of Electroencephalograph)
•UL Standard No 60601-1 (1st Edition) (Safety of Medical
Electrical Equipment, Part I: General Requirements for Safety)
•IEC 60601-2-26:2002 (Particular Requirements for the
Safety of Electroencephalograph)
•The GES 400 MR series systems carry the European CE mark
This medical equipment is classified as follows:
•Applied part: Type BF
•System: MDD Class IIa Equipment, Electrical Class I Equipment
IMPORTANT NOTICE: The GES 400 MR and GES 410 MR products are sold
for strictly research purposes and are not medical devices in the United
States. As such these products cannot be used for medical purposes such
as the diagnosis, treatment, cure, mitigation, or prevention of diseases.
The GES 400 MR and GES 410 MR products are CE marked in conformity
with the European Medical Device Directive. Information on clearance
status in other countries is available upon request.

18 GES 400 MR Series User Manual | 8100401-55 •2015-07-22
1.3.8 Rating
Table 1-6. Input ratings for GES MR components
Part Input Rating
GES External Power Supply 100-240 VAC, 50/60 Hz, 1.0 A
NA 400 series amplifier (from ext pwr sup) 12 VDC, 1.5 A
1.3.9 Interference
It is your responsibility to ensure that your GES MR system and its
components are safe and operate properly before using them.
All GES MR components have been tested and found to comply
with the electromagnetic compliance limits for the Medical Device
Directive 93/42/EEC (EN 60601-1-2:2007 Class A and JIS T 0601-1-
2:2002). See Appendix A.
These limits are designed to provide reasonable protection against
harmful interference in a typical medical installation. The
equipment generates radio-frequency energy and, if not installed
and used in accordance with the instructions, may cause
interference to other devices in the vicinity. If this equipment does
interfere with other devices, which can be determined by turning
the equipment off and on, try to correct the interference by one or
more of the following measures:
•Reorient or relocate the receiving device.
•Increase the separation between the equipment.
•Connect the equipment to an outlet on a different circuit
than the one used by the other devices.
•Consult EGI Technical Support (Table P-3).

GES 400 MR Series User Manual | 8100401-55 •2015-07-22 19
1.3.10 Symbols
The following symbols are used on GES MR components and in
this manual.
Table 1-7. Symbols used on GES MR components and in this manual
Symbol Description Symbol Description
MR conditional
MR unsafe
Imminent hazard
European conformity
Potential hazard
Canadian Standards
Association approval
Electrical hazard
Manufacturer
Type BF applied part
(body protected,
patient isolation)
EU authorized
representative
Interference hazard
Temperature limit
10˚C to 35˚C
(50˚F to 95˚F)
Functional earth
terminal
Humidity limits
Read product
documentation
(EGI and third-party mfr)
No unauthorized
servicing
Keep dry
Do not use in gas
environment
Always adhere to
IEC 60601-1-1 and
60601-1-2
Do not dispose with
other waste (comply
with local
regulations)
Signal input Digital inputs

20 GES 400 MR Series User Manual | 8100401-55 •2015-07-22
Symbol Description Symbol Description
Linked Part number
Active Serial number
Locked
Power input
Ethernet Powered
Clock sync Rx only Prescription medical
device
1.4 Safety Warnings
All EGI MR conditional equipment has been tested for MR safety based on the
guidelines from ASTM F2503-05 (Standard Practice for Marking Medical Devices
and Other Items for Safety in the Magnetic Resonance Environment), which is
one of the international standards of the American Society for Testing and
Materials (ASTM). Based on these tests (including displacement force and
torque, as well as RF heating), EGI’s MR conditional equipment is considered
safe in the specified MR environment.
The HC GSN MR Net is the only MR conditional device that will enter the bore of
a magnet. All related equipment is labeled as either MR conditional or MR
unsafe to ensure that only MR conditional devices will be allowed into the
specified MR environment. See the following tested use conditions.
This manual suits for next models
2
Table of contents
Other EGi Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Drainastim Pro
Drainastim Pro UFM001-1 user manual
Getinge
Getinge Arjohuntleigh Citadel Plus Instructions for use

BERNSTEIN
BERNSTEIN CS-3000 Installation and operating instructions

Bayer HealthCare
Bayer HealthCare Rapidlab 800 Operator's manual

Orliman
Orliman actius Instructions for use and care

Hospira
Hospira Lifecare PCA3 operating manual