ii Contents
430-04684-002
3) EQUIPMENT DESCRIPTION . . . . . . . . . . . . . . . . . . 3-1
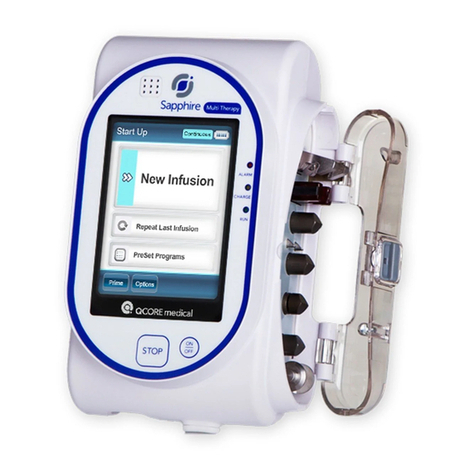
3.1 COMPONENTS . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
3.2 OPERATING BUTTONS & KEYS . . . . . . . . . . . . . . . 3-4
4) BASIC OPERATION . . . . . . . . . . . . . . . . . . . . . . . . 4-1
4.1 GETTING STARTED . . . . . . . . . . . . . . . . . . . . . . . 4-1
unpacking . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
connecting the patient pendant . . . . . . . . . . . . 4-1
system self-tests . . . . . . . . . . . . . . . . . . . . . . . . 4-2
data retention . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
4.2 OPERATING THE PCA 3 . . . . . . . . . . . . . . . . . . . 4-3
intravenous PCA administration . . . . . . . . . . . . . 4-3
epidural PCA administration . . . . . . . . . . . . . . . . 4-4
4.3 LOADING VIAL . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6
4.4 ADJUSTING SETTINGS ..................... 4-7
changing alarm volume . . . . . . . . . . . . . . . . . . . 4-8
changing contrast of main display . . . . . . . . . . . 4-9
changing or confirming time and date . . . . . . . . 4-9
4.5 GUIDED START-UP FOR PREFILLED VIALS . . . . . . 4-12
purging the system . . . . . . . . . . . . . . . . . . . . . 4-13
loading dose. . . . . . . . . . . . . . . . . . . . . . . . . . . 4-15
4.6 GUIDED START-UP FOR CUSTOM VIALS . . . . . . . 4-17
5) SELECT MODE . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
5.1 MODES OF DELIVERY . . . . . . . . . . . . . . . . . . . . . 5-1
protocols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
PCA only . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
continuous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-2
PCA+continuous . . . . . . . . . . . . . . . . . . . . . . . . 5-2
5.2 PROGRAMMING PCA ONLY ................. 5-3
5.3 CONTINUOUS MODE . . . . . . . . . . . . . . . . . . . . . . 5-7
5.4 PCA + CONTINUOUS MODE ............... 5-10
5.5 PROTOCOLS ........................... 5-16
5.6 DOSE LIMIT (1 OR 4 HOUR) . . . . . . . . . . . . . . . . 5-18
dose limit calculation . . . . . . . . . . . . . . . . . . . . 5-18
programming the 4 (or 1) hr dose limit. . . . . . . 5-20
to program a dose limit . . . . . . . . . . . . . . . . . . 5-20
to program NO dose limit . . . . . . . . . . . . . . . . . 5-21
PCA3NDC.book Page ii Tuesday, January 10, 2006 2:01 PM